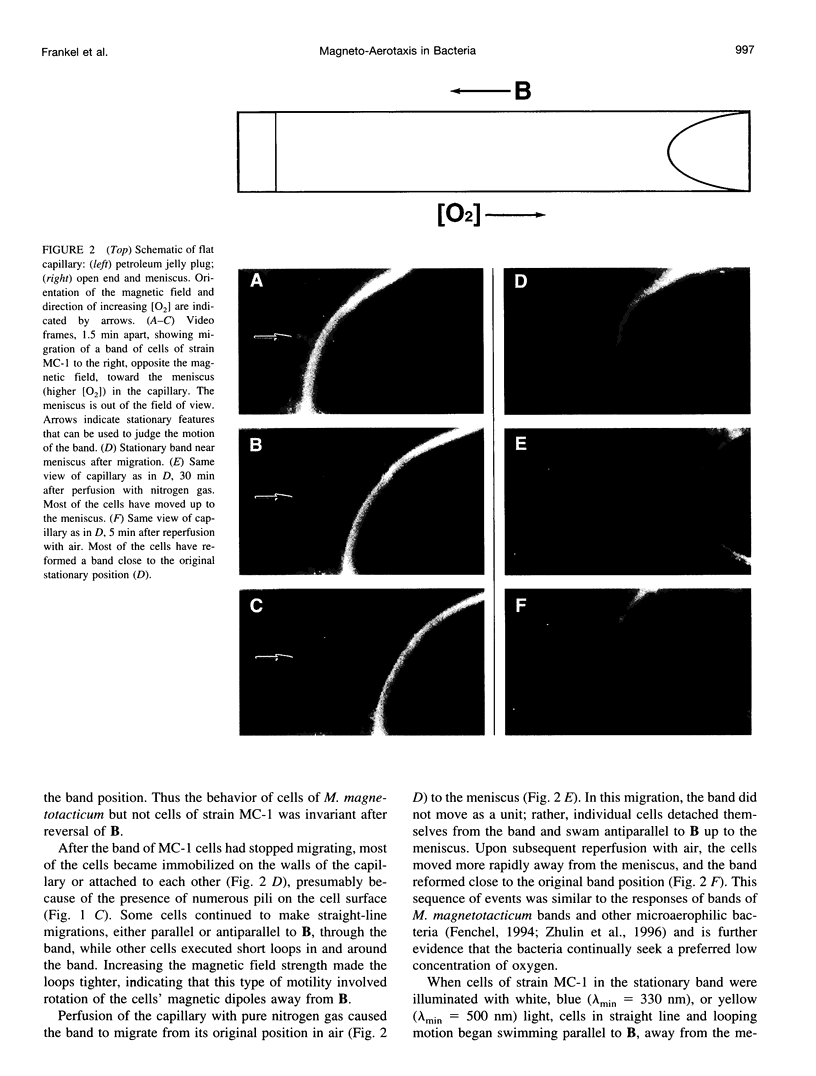
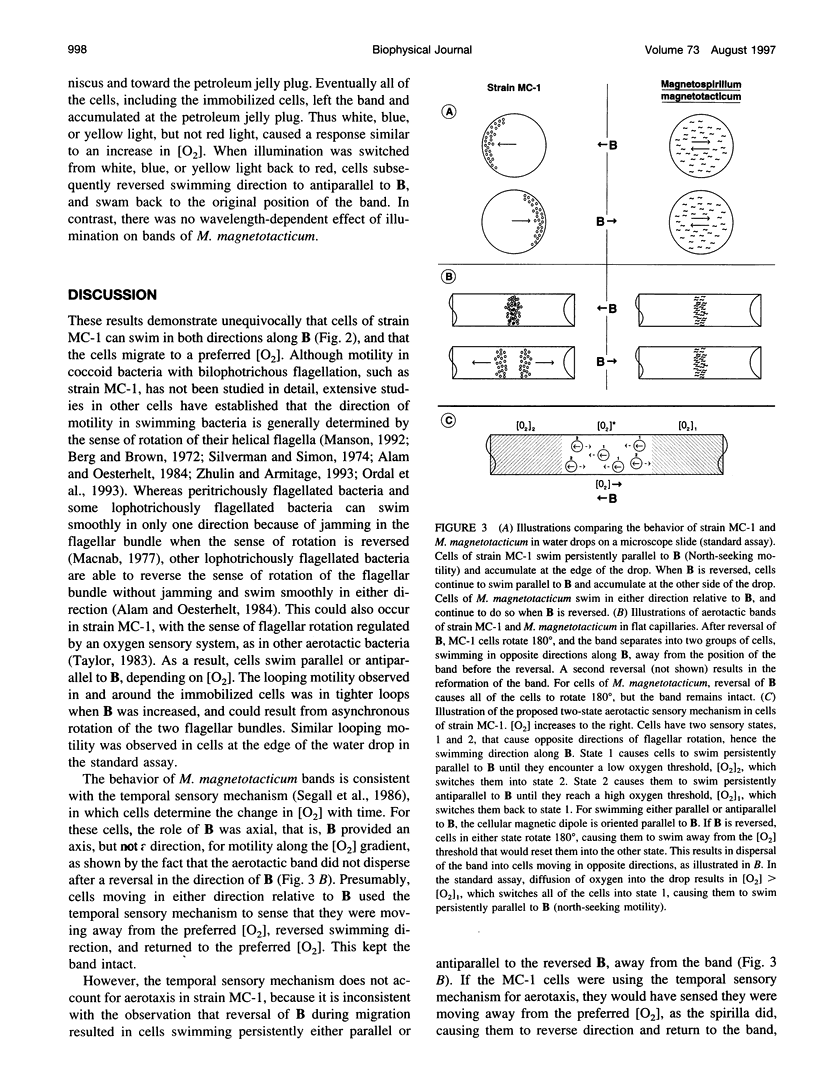
Abstract
Magnetotactic cocci swim persistently along local magnetic field lines in a preferred direction that corresponds to downward migration along geomagnetic field lines. Recently, high cell concentrations of magnetotactic cocci have been found in the water columns of chemically stratified, marine and brackish habitats, and not always in the sediments, as would be expected for persistent, downward-migrating bacteria. Here we report that cells of a pure culture of a marine magnetotactic coccus, designated strain MC-1, formed microaerophilic bands in capillary tubes and used aerotaxis to migrate to a preferred oxygen concentration in an oxygen gradient. Cells were able to swim in either direction along the local magnetic field and used magnetotaxis in conjunction with aerotaxis, i.e., magnetically assisted aerotaxis, or magneto-aerotaxis, to more efficiently migrate to and maintain position at their preferred oxygen concentration. Cells of strain MC-1 had a novel, aerotactic sensory mechanism that appeared to function as a two-way switch, rather than the temporal sensory mechanism used by other bacteria, including Magnetospirillum megnetotacticum, in aerotaxis. The cells also exhibited a response to short-wavelength light (< or = 500 nm), which caused them to swim persistently parallel to the magnetic field during illumination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam M., Oesterhelt D. Morphology, function and isolation of halobacterial flagella. J Mol Biol. 1984 Jul 15;176(4):459–475. doi: 10.1016/0022-2836(84)90172-4. [DOI] [PubMed] [Google Scholar]
- Bazylinski D. A., Frankel R. B., Heywood B. R., Mann S., King J. W., Donaghay P. L., Hanson A. K. Controlled Biomineralization of Magnetite (Fe(inf3)O(inf4)) and Greigite (Fe(inf3)S(inf4)) in a Magnetotactic Bacterium. Appl Environ Microbiol. 1995 Sep;61(9):3232–3239. doi: 10.1128/aem.61.9.3232-3239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazylinski D. A., Garratt-Reed A. J., Frankel R. B. Electron microscopic studies of magnetosomes in magnetotactic bacteria. Microsc Res Tech. 1994 Apr 1;27(5):389–401. doi: 10.1002/jemt.1070270505. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Chemotaxis in bacteria. Annu Rev Biophys Bioeng. 1975;4(00):119–136. doi: 10.1146/annurev.bb.04.060175.001003. [DOI] [PubMed] [Google Scholar]
- Bespalov V. A., Zhulin I. B., Taylor B. L. Behavioral responses of Escherichia coli to changes in redox potential. Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10084–10089. doi: 10.1073/pnas.93.19.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. P., Maratea D., Wolfe R. S. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol. 1979 Nov;140(2):720–729. doi: 10.1128/jb.140.2.720-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. Magnetotactic bacteria. Science. 1975 Oct 24;190(4212):377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- Delong E. F., Frankel R. B., Bazylinski D. A. Multiple evolutionary origins of magnetotaxis in bacteria. Science. 1993 Feb 5;259(5096):803–806. doi: 10.1126/science.259.5096.803. [DOI] [PubMed] [Google Scholar]
- Frankel R. B. Magnetic guidance of organisms. Annu Rev Biophys Bioeng. 1984;13:85–103. doi: 10.1146/annurev.bb.13.060184.000505. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Bacterial flagella rotating in bundles: a study in helical geometry. Proc Natl Acad Sci U S A. 1977 Jan;74(1):221–225. doi: 10.1073/pnas.74.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D. Bacterial motility and chemotaxis. Adv Microb Physiol. 1992;33:277–346. doi: 10.1016/s0065-2911(08)60219-2. [DOI] [PubMed] [Google Scholar]
- Moench T. T. Bilophococcus magnetotacticus gen. nov. sp. nov., a motile, magnetic coccus. Antonie Van Leeuwenhoek. 1988;54(6):483–496. doi: 10.1007/BF00588385. [DOI] [PubMed] [Google Scholar]
- Moench T. T., Konetzka W. A. A novel method for the isolation and study of a magnetotactic bacterium. Arch Microbiol. 1978 Nov 13;119(2):203–212. doi: 10.1007/BF00964274. [DOI] [PubMed] [Google Scholar]
- Segall J. E., Block S. M., Berg H. C. Temporal comparisons in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Spring S., Amann R., Ludwig W., Schleifer K. H., van Gemerden H., Petersen N. Dominating role of an unusual magnetotactic bacterium in the microaerobic zone of a freshwater sediment. Appl Environ Microbiol. 1993 Aug;59(8):2397–2403. doi: 10.1128/aem.59.8.2397-2403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain J. J. Models for teaching communication and attitudes. Cancer. 1982 Nov 1;50(9 Suppl):1974–1978. doi: 10.1002/1097-0142(19821101)50:9+<1974::aid-cncr2820501313>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Zhulin I. B., Armitage J. P. Motility, chemokinesis, and methylation-independent chemotaxis in Azospirillum brasilense. J Bacteriol. 1993 Feb;175(4):952–958. doi: 10.1128/jb.175.4.952-958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhulin I. B., Bespalov V. A., Johnson M. S., Taylor B. L. Oxygen taxis and proton motive force in Azospirillum brasilense. J Bacteriol. 1996 Sep;178(17):5199–5204. doi: 10.1128/jb.178.17.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]