Abstract
Binding of fluorescein-conjugated epidermal growth factor (EGF) to individual A431 cells at 4 degrees C is measured by a quantitative fluorescence imaging technique. After background fluorescence and cell autofluorescence photobleaching corrections, the kinetic data are fit to simple models of one monovalent site and two independent monovalent sites, both of which include a first-order dye photobleaching process. Model simulations and the results from data analysis indicate that the one-monovalent-site model does not describe EGF binding kinetics at the single-cell level, whereas the two-site model is consistent with, but not proved by, the single-cell binding data. In addition, the kinetics of binding of fluorescein-EGF to different cells from the same coverslip often differ significantly from each other, indicating cell-to-cell variations in the binding properties of the EGF receptor.
Full text
PDF


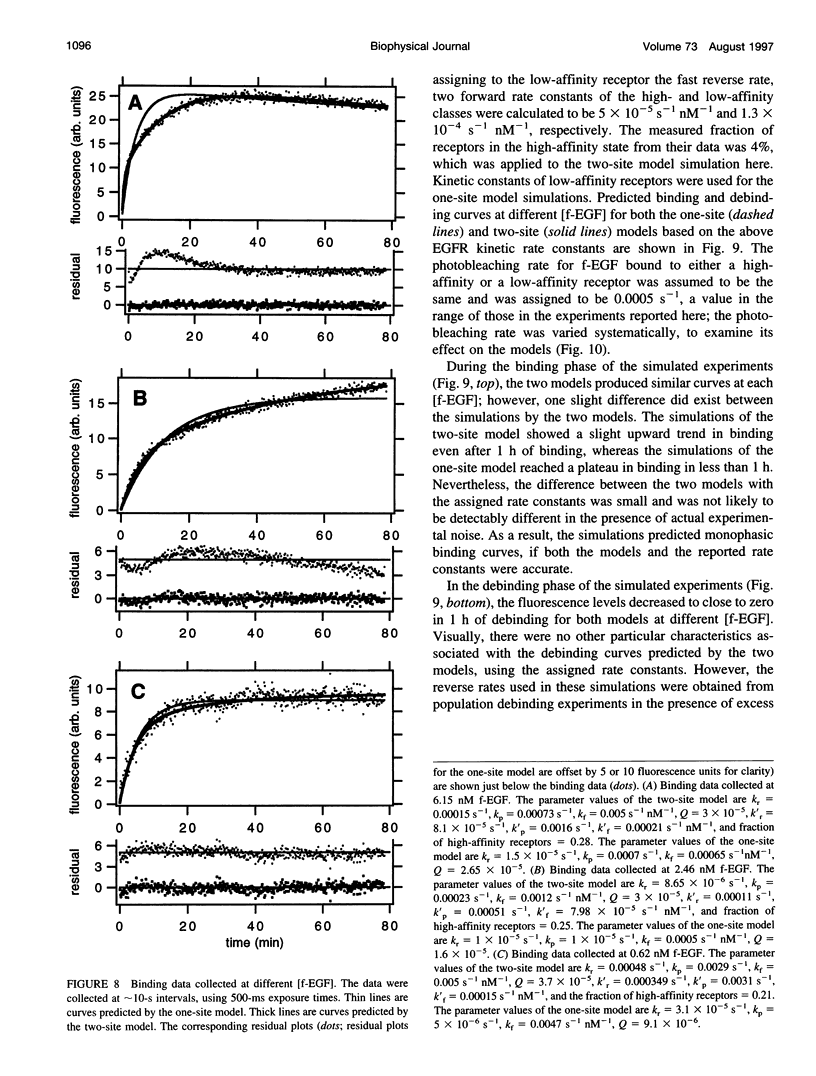
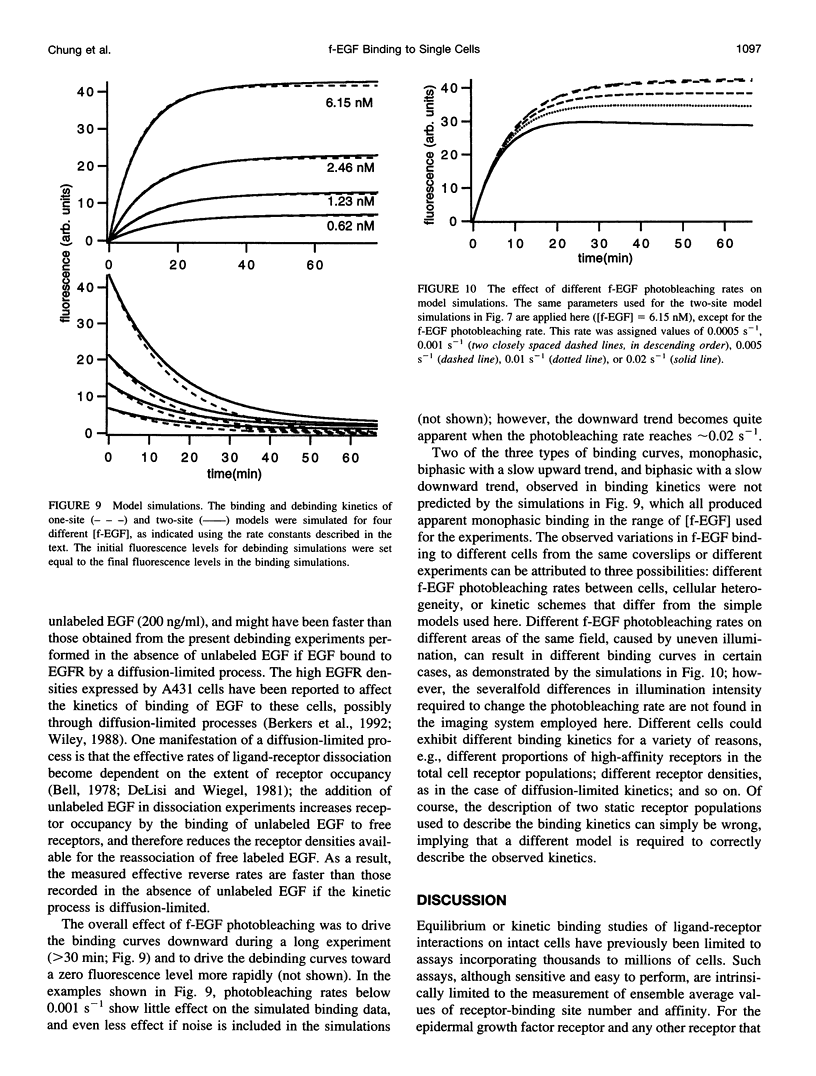





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Bellot F., Moolenaar W., Kris R., Mirakhur B., Verlaan I., Ullrich A., Schlessinger J., Felder S. High-affinity epidermal growth factor binding is specifically reduced by a monoclonal antibody, and appears necessary for early responses. J Cell Biol. 1990 Feb;110(2):491–502. doi: 10.1083/jcb.110.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkers J. A., van Bergen en Henegouwen P. P., Boonstra J. The effects of receptor density and cell shape on epidermal growth factor binding. J Recept Res. 1992;12(1):71–100. doi: 10.3109/10799899209066025. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Rijken P., Humbel B., Cremers F., Verkleij A., van Bergen en Henegouwen P. The epidermal growth factor. Cell Biol Int. 1995 May;19(5):413–430. doi: 10.1006/cbir.1995.1086. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., 3rd, Cerione R. A. Comparison of epidermal growth factor (EGF) receptor-receptor interactions in intact A431 cells and isolated plasma membranes. Large scale receptor micro-aggregation is not detected during EGF-stimulated early events. J Biol Chem. 1991 May 15;266(14):8899–8906. [PubMed] [Google Scholar]
- Chatelier R. C., Ashcroft R. G., Lloyd C. J., Nice E. C., Whitehead R. H., Sawyer W. H., Burgess A. W. Binding of fluoresceinated epidermal growth factor to A431 cell sub-populations studied using a model-independent analysis of flow cytometric fluorescence data. EMBO J. 1986 Jun;5(6):1181–1186. doi: 10.1002/j.1460-2075.1986.tb04344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyette T. E., Gross D. J. Epidermal growth factor-stimulated calcium ion transients in individual A431 cells: initiation kinetics and ligand concentration dependence. Cell Regul. 1991 Oct;2(10):827–840. doi: 10.1091/mbc.2.10.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M., McKanna J. A., Cohen S. Rapid induction of morphological changes in human carcinoma cells A-431 by epidermal growth factors. J Cell Biol. 1979 Oct;83(1):260–265. doi: 10.1083/jcb.83.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M., McKanna J. A., Cohen S. Rapid rounding of human epidermoid carcinoma cells A-431 induced by epidermal growth factor. J Cell Biol. 1981 Feb;88(2):422–429. doi: 10.1083/jcb.88.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Soderquist A. M., Carpenter G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J Biol Chem. 1985 Oct 5;260(22):11944–11952. [PubMed] [Google Scholar]
- Davis R. J., Gironès N., Faucher M. Two alternative mechanisms control the interconversion of functional states of the epidermal growth factor receptor. J Biol Chem. 1988 Apr 15;263(11):5373–5379. [PubMed] [Google Scholar]
- DeLisi C., Wiegel F. W. Effect of nonspecific forces and finite receptor number on rate constants of ligand--cell bound-receptor interactions. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5569–5572. doi: 10.1073/pnas.78.9.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defize L. H., Boonstra J., Meisenhelder J., Kruijer W., Tertoolen L. G., Tilly B. C., Hunter T., van Bergen en Henegouwen P. M., Moolenaar W. H., de Laat S. W. Signal transduction by epidermal growth factor occurs through the subclass of high affinity receptors. J Cell Biol. 1989 Nov;109(5):2495–2507. doi: 10.1083/jcb.109.5.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadella T. W., Jr, Jovin T. M. Oligomerization of epidermal growth factor receptors on A431 cells studied by time-resolved fluorescence imaging microscopy. A stereochemical model for tyrosine kinase receptor activation. J Cell Biol. 1995 Jun;129(6):1543–1558. doi: 10.1083/jcb.129.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Gregorou M., Rees A. R. Properties of a monoclonal antibody to epidermal growth factor receptor with implications for the mechanism of action of EGF. EMBO J. 1984 May;3(5):929–937. doi: 10.1002/j.1460-2075.1984.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman G. M., Schlessinger J. Lateral diffusion of epidermal growth factor complexed to its surface receptors does not account for the thermal sensitivity of patch formation and endocytosis. Biochemistry. 1982 Mar 30;21(7):1667–1672. doi: 10.1021/bi00536a030. [DOI] [PubMed] [Google Scholar]
- Lax I., Bellot F., Howk R., Ullrich A., Givol D., Schlessinger J. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J. 1989 Feb;8(2):421–427. doi: 10.1002/j.1460-2075.1989.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I., Burgess W. H., Bellot F., Ullrich A., Schlessinger J., Givol D. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol. 1988 Apr;8(4):1831–1834. doi: 10.1128/mcb.8.4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Bu Z., Ladbury J. E., Zhou M., Pinchasi D., Lax I., Engelman D. M., Schlessinger J. Two EGF molecules contribute additively to stabilization of the EGFR dimer. EMBO J. 1997 Jan 15;16(2):281–294. doi: 10.1093/emboj/16.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994 Nov;19(11):459–463. doi: 10.1016/0968-0004(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Linderman J. J., Harris L. J., Slakey L. L., Gross D. J. Charge-coupled device imaging of rapid calcium transients in cultured arterial smooth muscle cells. Cell Calcium. 1990 Feb-Mar;11(2-3):131–144. doi: 10.1016/0143-4160(90)90066-4. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994 Jul;126(1):111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui H., Castro L., Mendelsohn J. Consumption of EGF by A431 cells: evidence for receptor recycling. J Cell Biol. 1993 Jan;120(1):85–93. doi: 10.1083/jcb.120.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes E. L., Waterfield M. D. Biosynthesis of the epidermal growth factor receptor in A431 cells. EMBO J. 1984 Mar;3(3):531–537. doi: 10.1002/j.1460-2075.1984.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna J. A., Haigler H. T., Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K., Beardmore J., Kanety H., Schlessinger J., Hopkins C. R. Localization of the epidermal growth factor (EGF) receptor within the endosome of EGF-stimulated epidermoid carcinoma (A431) cells. J Cell Biol. 1986 Feb;102(2):500–509. doi: 10.1083/jcb.102.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C., James G. L., Smart E. J., Anderson R. G. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996 May 17;271(20):11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Northwood I. C., Davis R. J. Protein kinase C inhibition of the epidermal growth factor receptor tyrosine protein kinase activity is independent of the oligomeric state of the receptor. J Biol Chem. 1989 Apr 5;264(10):5746–5750. [PubMed] [Google Scholar]
- Rees A. R., Gregoriou M., Johnson P., Garland P. B. High affinity epidermal growth factor receptors on the surface of A431 cells have restricted lateral diffusion. EMBO J. 1984 Aug;3(8):1843–1847. doi: 10.1002/j.1460-2075.1984.tb02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988 Nov;13(11):443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- Sherrill J. M., Kyte J. Activation of epidermal growth factor receptor by epidermal growth factor. Biochemistry. 1996 May 7;35(18):5705–5718. doi: 10.1021/bi9602268. [DOI] [PubMed] [Google Scholar]
- Slieker L. J., Lane M. D. Post-translational processing of the epidermal growth factor receptor. Glycosylation-dependent acquisition of ligand-binding capacity. J Biol Chem. 1985 Jan 25;260(2):687–690. [PubMed] [Google Scholar]
- Smart E. J., Ying Y. S., Mineo C., Anderson R. G. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Hennink E. J., Young I. T., Tanke H. J. Photobleaching kinetics of fluorescein in quantitative fluorescence microscopy. Biophys J. 1995 Jun;68(6):2588–2600. doi: 10.1016/S0006-3495(95)80442-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Mitchell W. M., Cohen S. Epidermal growth factor. Physical and chemical properties. J Biol Chem. 1972 Sep 25;247(18):5928–5934. [PubMed] [Google Scholar]
- Tomoda H., Kishimoto Y., Lee Y. C. Temperature effect on endocytosis and exocytosis by rabbit alveolar macrophages. J Biol Chem. 1989 Sep 15;264(26):15445–15450. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Weber W., Bertics P. J., Gill G. N. Immunoaffinity purification of the epidermal growth factor receptor. Stoichiometry of binding and kinetics of self-phosphorylation. J Biol Chem. 1984 Dec 10;259(23):14631–14636. [PubMed] [Google Scholar]
- Weber W., Gill G. N., Spiess J. Production of an epidermal growth factor receptor-related protein. Science. 1984 Apr 20;224(4646):294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J Biol Chem. 1981 Mar 25;256(6):2615–2617. [PubMed] [Google Scholar]
- Wiley H. S. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988 Aug;107(2):801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann M. M., Fox C. F. Identification of epidermal growth factor receptors in a hyperproducing human epidermoid carcinoma cell line. J Biol Chem. 1979 Sep 10;254(17):8083–8086. [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987 Mar 10;26(5):1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- den Hartigh J. C., van Bergen en Henegouwen P. M., Verkleij A. J., Boonstra J. The EGF receptor is an actin-binding protein. J Cell Biol. 1992 Oct;119(2):349–355. doi: 10.1083/jcb.119.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belzen N., Rijken P. J., Verkleij A. J., Boonstra J. Sulfhydryl reagents alter epidermal growth factor receptor affinity and association with the cytoskeleton. J Recept Res. 1991;11(6):919–940. doi: 10.3109/10799899109064688. [DOI] [PubMed] [Google Scholar]
- van Bergen en Henegouwen P. M., Defize L. H., de Kroon J., van Damme H., Verkleij A. J., Boonstra J. Ligand-induced association of epidermal growth factor receptor to the cytoskeleton of A431 cells. J Cell Biochem. 1989 Apr;39(4):455–465. doi: 10.1002/jcb.240390411. [DOI] [PubMed] [Google Scholar]
- van Bergen en Henegouwen P. M., den Hartigh J. C., Romeyn P., Verkleij A. J., Boonstra J. The epidermal growth factor receptor is associated with actin filaments. Exp Cell Res. 1992 Mar;199(1):90–97. doi: 10.1016/0014-4827(92)90465-k. [DOI] [PubMed] [Google Scholar]


