Abstract
The determination of complete electrorotation spectra of living cells has been made possible by the development of a quadrature generator and an electrode assembly that span the frequency range between 100 Hz and 1.6 GHz. Multiple spectra of single cells of the yeast Saccharomyces cerevisiae have been measured at different medium conductivities ranging from 0.7 to 550 microS cm-1. A spherical four-shell model was applied that simulated the experimental data well and disclosed the four-layer structure of the cell envelope attributed to the plasma membrane, the periplasmic space, and a thick inner and a thin outer wall region. Below 10 kHz an additional rotation effect was found, which changed its direction depending on the ionic strength of the medium. This is supposed to be connected with properties of the cell surface and its close vicinity. From the four-shell simulation the following physical properties of cell compartments could be derived: specific capacitance of plasma membrane (0.76 microF cm-2), periplasmic space (0.5 microF cm-2), and outer wall region (0.1 microF cm-2). The conductivity of cytoplasm, plasma membrane, and inner wall region were found to vary with medium ionic strength from 9 to 12 mS cm-1, 5.8 nS cm-1 to approximately 50 nS cm-1, and 6 microS cm-1 to 240 microS cm-1, respectively.
Full text
PDF
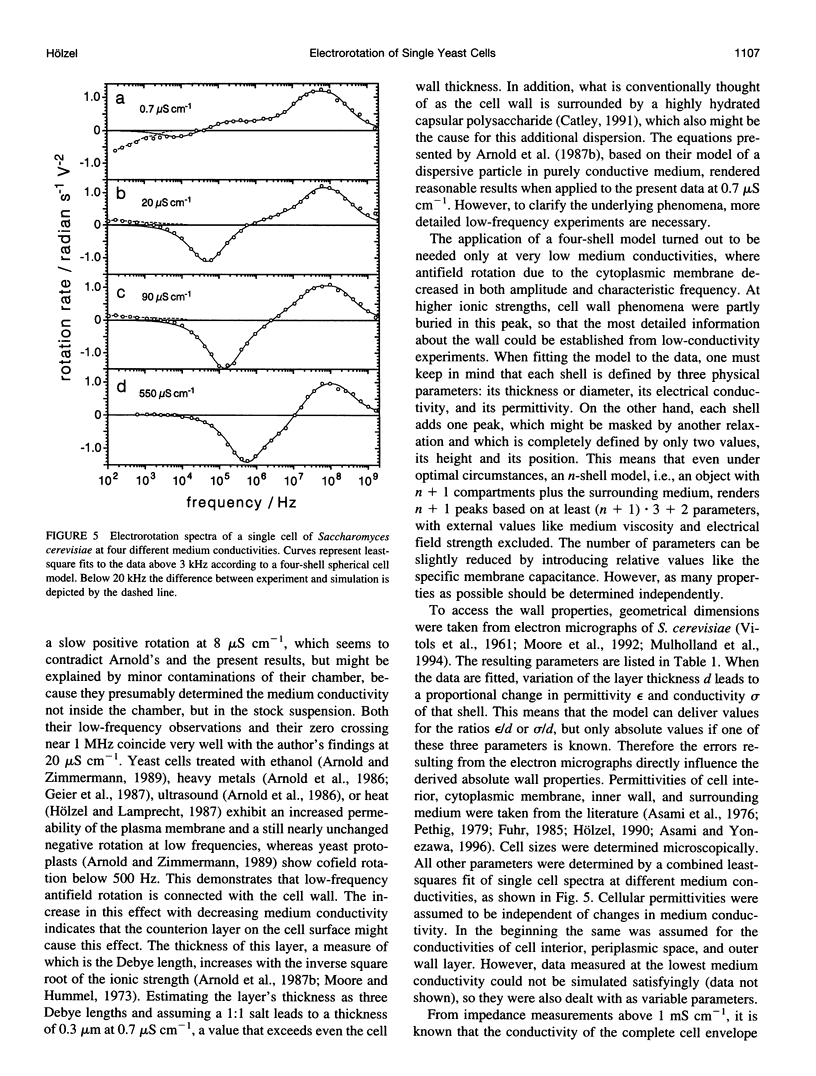
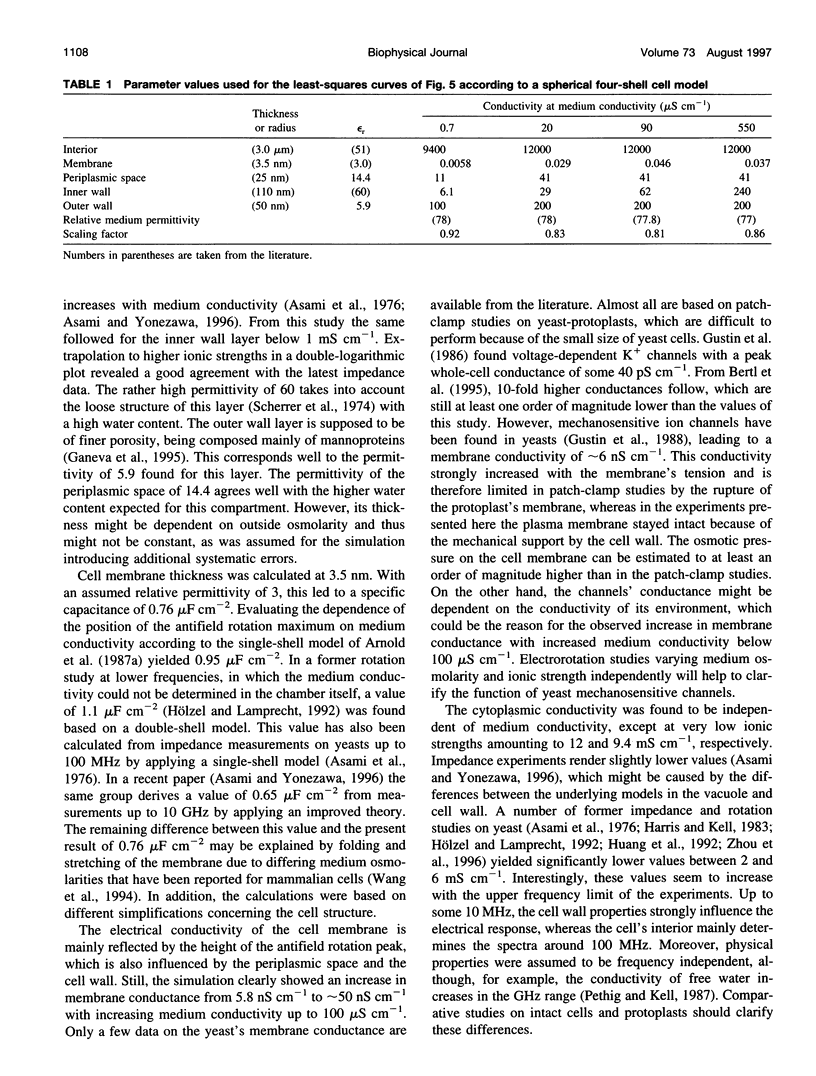

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. M., Schmutzler R. K., Schmutzler A. G., van der Ven H., Al-Hasani S., Krebs D., Zimmermann U. Electro-rotation of mouse oocytes: single-cell measurements of zona-intact and zona-free cells and of the isolated zona pellucida. Biochim Biophys Acta. 1987 Dec 11;905(2):454–464. doi: 10.1016/0005-2736(87)90475-5. [DOI] [PubMed] [Google Scholar]
- Arnold W. N., Lacy J. S. Permeability of the cell envelope and osmotic behavior in Saccharomyces cerevisiae. J Bacteriol. 1977 Aug;131(2):564–571. doi: 10.1128/jb.131.2.564-571.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami K., Hanai T., Koizumi N. Dielectric properties of yeast cells. J Membr Biol. 1976 Aug 26;28(2-3):169–180. doi: 10.1007/BF01869695. [DOI] [PubMed] [Google Scholar]
- Asami K., Yonezawa T. Dielectric behavior of wild-type yeast and vacuole-deficient mutant over a frequency range of 10 kHz to 10 GHz. Biophys J. 1996 Oct;71(4):2192–2200. doi: 10.1016/S0006-3495(96)79420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A., Anderson J. A., Slayman C. L., Gaber R. F. Use of Saccharomyces cerevisiae for patch-clamp analysis of heterologous membrane proteins: characterization of Kat1, an inward-rectifying K+ channel from Arabidopsis thaliana, and comparison with endogeneous yeast channels and carriers. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2701–2705. doi: 10.1073/pnas.92.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr G., Glasser H., Müller T., Schnelle T. Cell manipulation and cultivation under a.c. electric field influence in highly conductive culture media. Biochim Biophys Acta. 1994 Dec 15;1201(3):353–360. doi: 10.1016/0304-4165(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Ganeva V., Galutzov B., Teissié J. Electric field mediated loading of macromolecules in intact yeast cells is critically controlled at the wall level. Biochim Biophys Acta. 1995 Dec 13;1240(2):229–236. doi: 10.1016/0005-2736(95)00181-6. [DOI] [PubMed] [Google Scholar]
- Geier B. M., Wendt B., Arnold W. M., Zimmermann U. The effect of mercuric salts on the electro-rotation of yeast cells and comparison with a theoretical model. Biochim Biophys Acta. 1987 Jun 12;900(1):45–55. doi: 10.1016/0005-2736(87)90276-8. [DOI] [PubMed] [Google Scholar]
- Gimsa J., Marszalek P., Loewe U., Tsong T. Y. Dielectrophoresis and electrorotation of neurospora slime and murine myeloma cells. Biophys J. 1991 Oct;60(4):749–760. doi: 10.1016/S0006-3495(91)82109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa J., Müller T., Schnelle T., Fuhr G. Dielectric spectroscopy of single human erythrocytes at physiological ionic strength: dispersion of the cytoplasm. Biophys J. 1996 Jul;71(1):495–506. doi: 10.1016/S0006-3495(96)79251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Martinac B., Saimi Y., Culbertson M. R., Kung C. Ion channels in yeast. Science. 1986 Sep 12;233(4769):1195–1197. doi: 10.1126/science.2426783. [DOI] [PubMed] [Google Scholar]
- Gustin M. C., Zhou X. L., Martinac B., Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988 Nov 4;242(4879):762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- Huang Y., Hölzel R., Pethig R., Wang X. B. Differences in the AC electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Phys Med Biol. 1992 Jul;37(7):1499–1517. doi: 10.1088/0031-9155/37/7/003. [DOI] [PubMed] [Google Scholar]
- Hölzel R., Lamprecht I. Cellular spin resonance of yeast in a frequency range up to 140 MHz. Z Naturforsch C. 1987 Nov-Dec;42(11-12):1367–1369. doi: 10.1515/znc-1987-11-1242. [DOI] [PubMed] [Google Scholar]
- Hölzel R., Lamprecht I. Dielectric properties of yeast cells as determined by electrorotation. Biochim Biophys Acta. 1992 Feb 17;1104(1):195–200. doi: 10.1016/0005-2736(92)90150-k. [DOI] [PubMed] [Google Scholar]
- Moore C. W., Del Valle R., McKoy J., Pramanik A., Gordon R. E. Lesions and preferential initial localization of [S-methyl-3H]bleomycin A2 on Saccharomyces cerevisiae cell walls and membranes. Antimicrob Agents Chemother. 1992 Nov;36(11):2497–2505. doi: 10.1128/aac.36.11.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J., Preuss D., Moon A., Wong A., Drubin D., Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994 Apr;125(2):381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethig R., Kell D. B. The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Phys Med Biol. 1987 Aug;32(8):933–970. doi: 10.1088/0031-9155/32/8/001. [DOI] [PubMed] [Google Scholar]
- Scherrer R., Louden L., Gerhardt P. Porosity of the yeast cell wall and membrane. J Bacteriol. 1974 May;118(2):534–540. doi: 10.1128/jb.118.2.534-540.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VITOLS E., NORTH R. J., LINNANE A. W. Studies on the oxidative metabolism of Saccharomyces cerevisiae. I. Observations on the fine structure of the yeast cell. J Biophys Biochem Cytol. 1961 Mar;9:689–699. doi: 10.1083/jcb.9.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. B., Huang Y., Gascoyne P. R., Becker F. F., Hölzel R., Pethig R. Changes in Friend murine erythroleukaemia cell membranes during induced differentiation determined by electrorotation. Biochim Biophys Acta. 1994 Aug 3;1193(2):330–344. doi: 10.1016/0005-2736(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Markx G. H., Pethig R. Effect of biocide concentration on electrorotation spectra of yeast cells. Biochim Biophys Acta. 1996 May 22;1281(1):60–64. doi: 10.1016/0005-2736(96)00015-6. [DOI] [PubMed] [Google Scholar]