Abstract
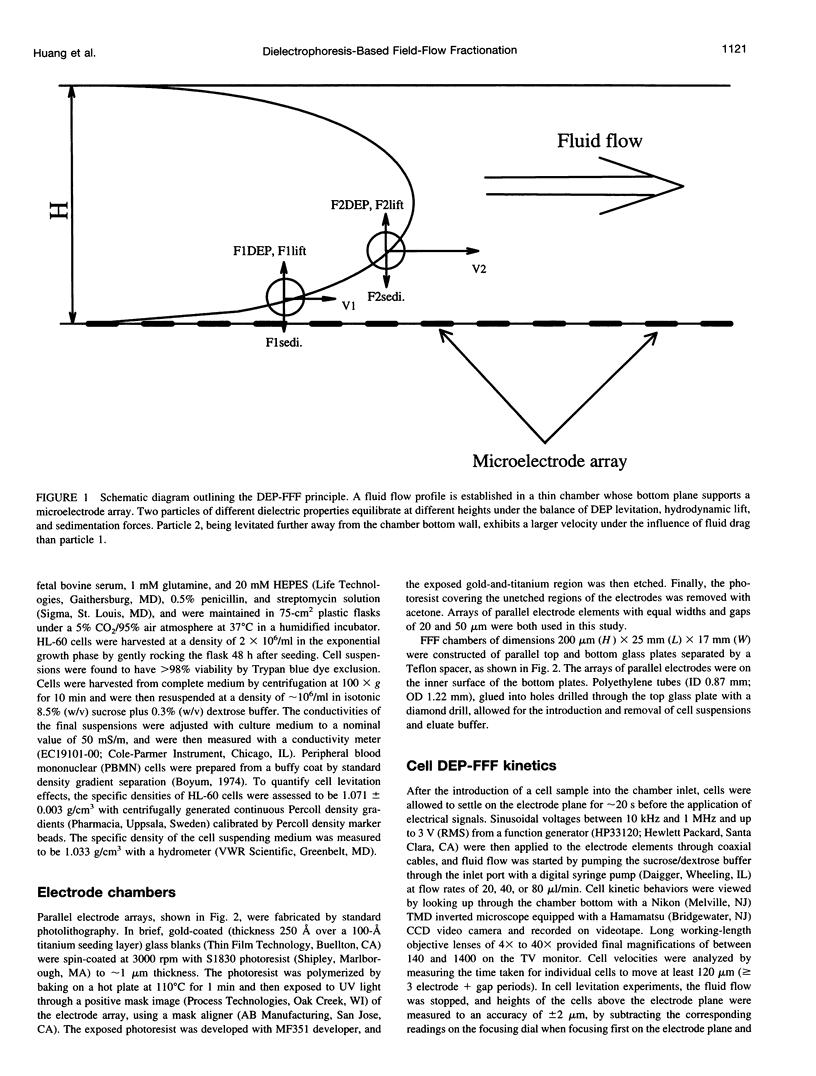
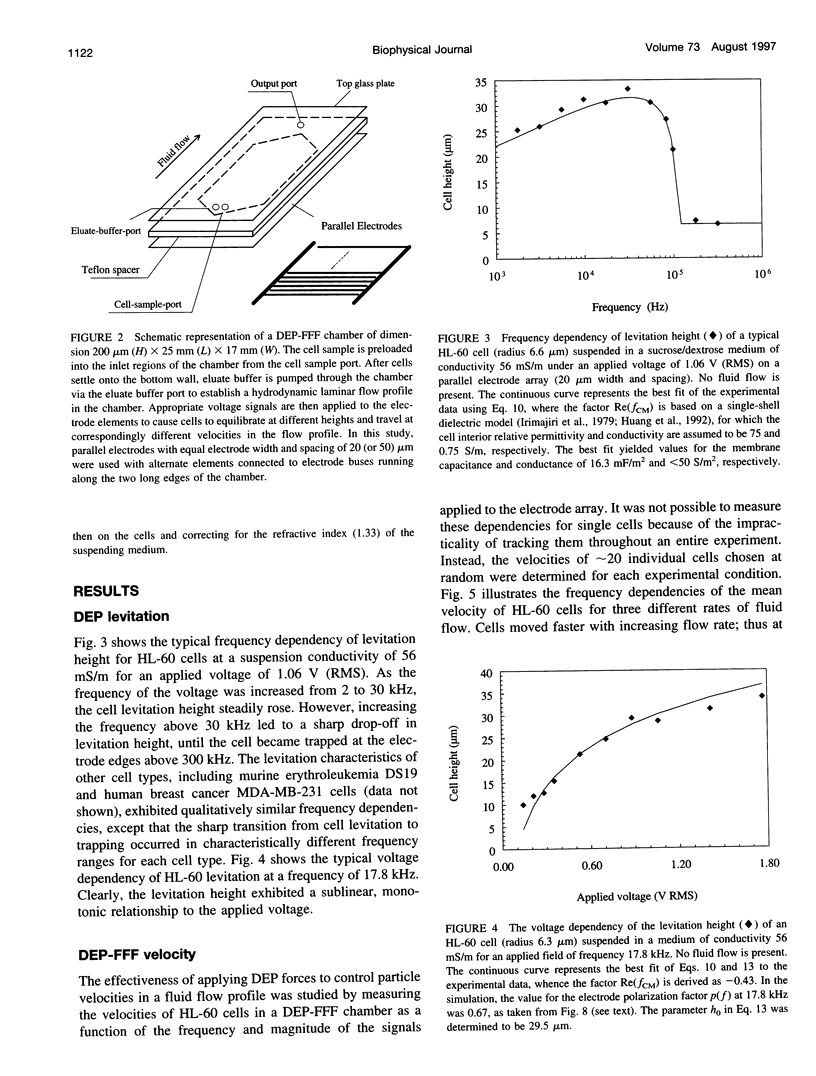
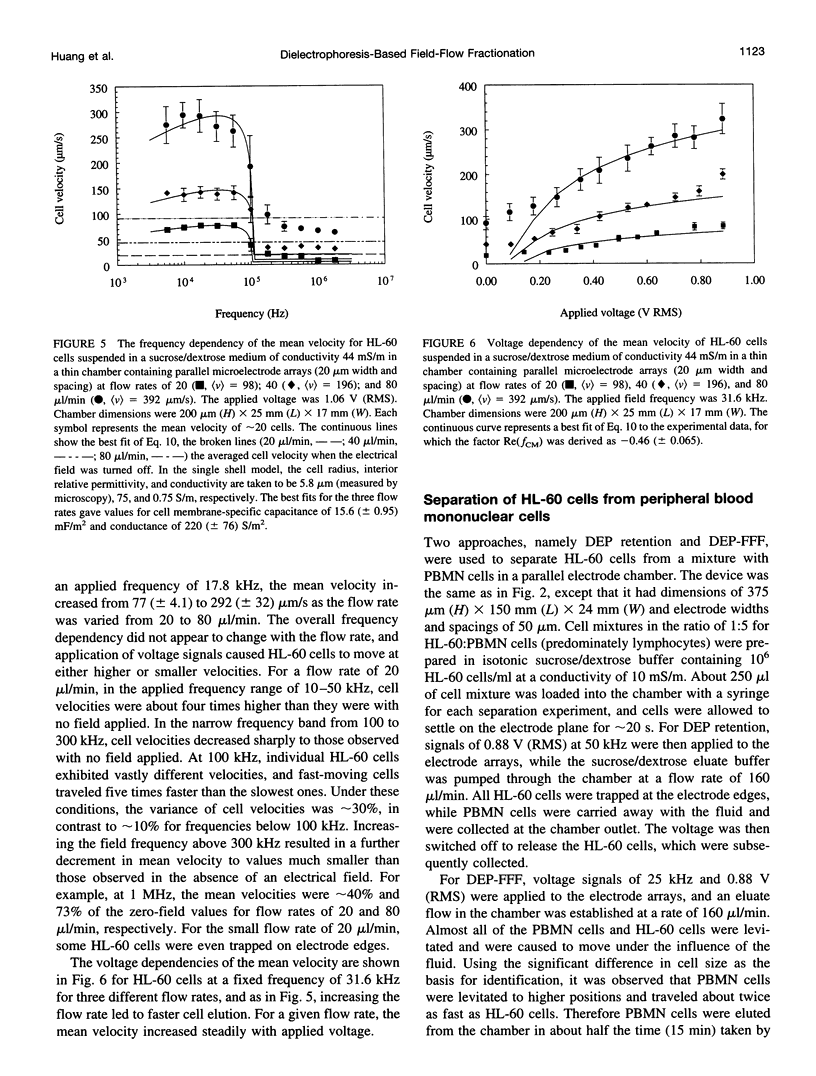
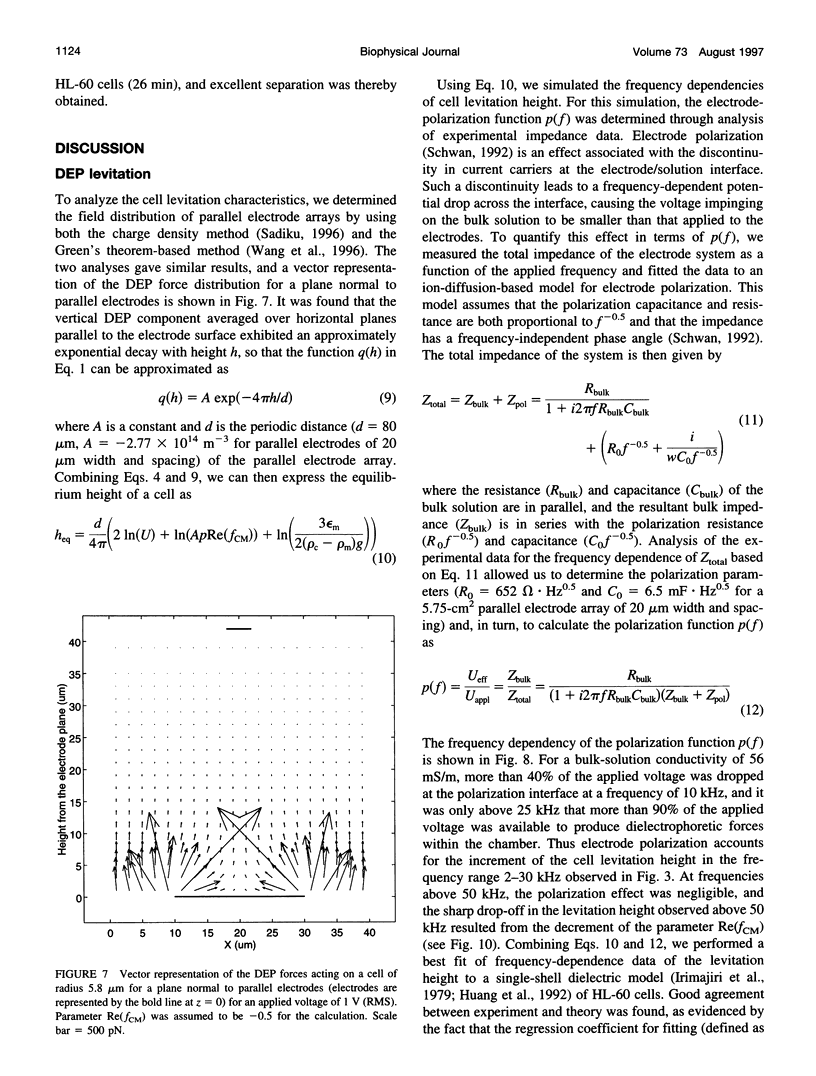
We present the principle of cell characterization and separation by dielectrophoretic field-flow fractionation and show preliminary experimental results. The operational device takes the form of a thin chamber in which the bottom wall supports an array of microelectrodes. By applying appropriate AC voltage signals to these electrodes, dielectrophoretic forces are generated to levitate cells suspended in the chamber and to affect their equilibrium heights. A laminar flow profile is established in the chamber so that fluid flows faster with increasing distance from the chamber walls. A cell carried in the flow stream will attain an equilibrium height, and a corresponding velocity, based on the balance of dielectrophoretic, gravitational, and hydrodynamic lift forces it experiences. We describe a theoretical model for this system and show that the cell velocity is a function of the mean fluid velocity, the voltage and frequency of the signals applied to the electrodes, and, most significantly, the cell dielectric properties. The validity of the model is demonstrated with human leukemia (HL-60) cells subjected to a parallel electrode array, and application of the device to separating HL-60 cells from peripheral blood mononuclear cells is shown.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreux J. P., Merino A., Renard M., Forestier F., Cardot P. Separation of red blood cells by field flow fractionation. Exp Hematol. 1993 Feb;21(2):326–330. [PubMed] [Google Scholar]
- Becker F. F., Wang X. B., Huang Y., Pethig R., Vykoukal J., Gascoyne P. R. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):860–864. doi: 10.1073/pnas.92.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Selection of motile nonchemotactic mutants of Escherichia coli by field-flow fractionation. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8145–8148. doi: 10.1073/pnas.88.18.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Cardot P. J., Elgéa C., Guernet M., Godet D., Andreux J. P. Size- and density-dependent elution of normal and pathological red blood cells by gravitational field-flow fractionation. J Chromatogr B Biomed Appl. 1994 Apr 1;654(2):193–203. doi: 10.1016/0378-4347(94)00011-5. [DOI] [PubMed] [Google Scholar]
- Fischer A. The use of monoclonal antibodies in allogeneic bone marrow transplantation. Br J Haematol. 1993 Apr;83(4):531–534. doi: 10.1111/j.1365-2141.1993.tb04686.x. [DOI] [PubMed] [Google Scholar]
- Fuhr G., Arnold W. M., Hagedorn R., Müller T., Benecke W., Wagner B., Zimmermann U. Levitation, holding, and rotation of cells within traps made by high-frequency fields. Biochim Biophys Acta. 1992 Jul 27;1108(2):215–223. doi: 10.1016/0005-2736(92)90028-k. [DOI] [PubMed] [Google Scholar]
- Giddings J. C. Field-flow fractionation of macromolecules. J Chromatogr. 1989 May 26;470(2):327–335. doi: 10.1016/s0021-9673(01)83561-5. [DOI] [PubMed] [Google Scholar]
- Giddings J. C. Field-flow fractionation: analysis of macromolecular, colloidal, and particulate materials. Science. 1993 Jun 4;260(5113):1456–1465. doi: 10.1126/science.8502990. [DOI] [PubMed] [Google Scholar]
- Huang Y., Hölzel R., Pethig R., Wang X. B. Differences in the AC electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Phys Med Biol. 1992 Jul;37(7):1499–1517. doi: 10.1088/0031-9155/37/7/003. [DOI] [PubMed] [Google Scholar]
- Irimajiri A., Hanai T., Inouye A. A dielectric theory of "multi-stratified shell" model with its application to a lymphoma cell. J Theor Biol. 1979 May 21;78(2):251–269. doi: 10.1016/0022-5193(79)90268-6. [DOI] [PubMed] [Google Scholar]
- Kaler K. V., Jones T. B. Dielectrophoretic spectra of single cells determined by feedback-controlled levitation. Biophys J. 1990 Feb;57(2):173–182. doi: 10.1016/S0006-3495(90)82520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzén A., Walter J. K., Krischollek H., Wahlund K. G. Separation and quantitation of monoclonal antibody aggregates by asymmetrical flow field-flow fractionation and comparison to gel permeation chromatography. Anal Biochem. 1993 Aug 1;212(2):469–480. doi: 10.1006/abio.1993.1356. [DOI] [PubMed] [Google Scholar]
- Markx G. H., Talary M. S., Pethig R. Separation of viable and non-viable yeast using dielectrophoresis. J Biotechnol. 1994 Jan 15;32(1):29–37. doi: 10.1016/0168-1656(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Mozersky S. M., Caldwell K. D., Jones S. B., Maleeff B. E., Barford R. A. Sedimentation field flow fractionation of mitochondrial and microsomal membranes from corn roots. Anal Biochem. 1988 Jul;172(1):113–123. doi: 10.1016/0003-2697(88)90419-8. [DOI] [PubMed] [Google Scholar]
- Schwan H. P. Linear and nonlinear electrode polarization and biological materials. Ann Biomed Eng. 1992;20(3):269–288. doi: 10.1007/BF02368531. [DOI] [PubMed] [Google Scholar]
- Smeland E. B., Funderud S., Kvalheim G., Gaudernack G., Rasmussen A. M., Rusten L., Wang M. Y., Tindle R. W., Blomhoff H. K., Egeland T. Isolation and characterization of human hematopoietic progenitor cells: an effective method for positive selection of CD34+ cells. Leukemia. 1992 Aug;6(8):845–852. [PubMed] [Google Scholar]
- Talary M. S., Mills K. I., Hoy T., Burnett A. K., Pethig R. Dielectrophoretic separation and enrichment of CD34+ cell subpopulation from bone marrow and peripheral blood stem cells. Med Biol Eng Comput. 1995 Mar;33(2):235–237. doi: 10.1007/BF02523050. [DOI] [PubMed] [Google Scholar]
- Wang X. B., Huang Y., Gascoyne P. R., Becker F. F., Hölzel R., Pethig R. Changes in Friend murine erythroleukaemia cell membranes during induced differentiation determined by electrorotation. Biochim Biophys Acta. 1994 Aug 3;1193(2):330–344. doi: 10.1016/0005-2736(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Wang X. B., Huang Y., Wang X., Becker F. F., Gascoyne P. R. Dielectrophoretic manipulation of cells with spiral electrodes. Biophys J. 1997 Apr;72(4):1887–1899. doi: 10.1016/S0006-3495(97)78834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. B., Hughes M. P., Huang Y., Becker F. F., Gascoyne P. R. Non-uniform spatial distributions of both the magnitude and phase of AC electric fields determine dielectrophoretic forces. Biochim Biophys Acta. 1995 Feb 23;1243(2):185–194. doi: 10.1016/0304-4165(94)00146-o. [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Markx G. H., Pethig R., Eastwood I. M. Differentiation of viable and non-viable bacterial biofilms using electrorotation. Biochim Biophys Acta. 1995 Aug 17;1245(1):85–93. doi: 10.1016/0304-4165(95)00072-j. [DOI] [PubMed] [Google Scholar]


