Abstract
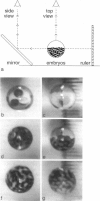
We have levitated, for the first time, living biological specimens, embryos of the frog Xenopus laevis, using a large inhomogeneous magnetic field. The magnetic field/field gradient product required for levitation was 1430 kG2/cm, consistent with the embryo's susceptibility being dominated by the diamagnetism of water and protein. We show that unlike any other earth-based technique, magnetic field gradient levitation of embryos reduces the body forces and gravity-induced stresses on them. We discuss the use of large inhomogeneous magnetic fields as a probe for gravitationally sensitive phenomena in biological specimens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. The simulation of microgravity conditions on the ground. ASGSB Bull. 1992 Oct;5(2):3–10. [PubMed] [Google Scholar]
- Barone R. P., Caren L. D. The immune system: effects of hypergravity and hypogravity. Aviat Space Environ Med. 1984 Nov;55(11):1063–1068. [PubMed] [Google Scholar]
- Black S. D., Gerhart J. C. Experimental control of the site of embryonic axis formation in Xenopus laevis eggs centrifuged before first cleavage. Dev Biol. 1985 Apr;108(2):310–324. doi: 10.1016/0012-1606(85)90035-1. [DOI] [PubMed] [Google Scholar]
- Brandt E. H. Levitation in physics. Science. 1989 Jan 20;243(4889):349–355. doi: 10.1126/science.243.4889.349. [DOI] [PubMed] [Google Scholar]
- Cooke J. Permanent distortion of positional system of Xenopus embryo by brief early perturbation in gravity. Nature. 1986 Jan 2;319(6048):60–63. doi: 10.1038/319060a0. [DOI] [PubMed] [Google Scholar]
- Denegre J. M., Danilchik M. V. Deep cytoplasmic rearrangements in axis-respecified Xenopus embryos. Dev Biol. 1993 Nov;160(1):157–164. doi: 10.1006/dbio.1993.1294. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Ubbels G., Black S., Hara K., Kirschner M. A reinvestigation of the role of the grey crescent in axis formation in xenopus laevis. Nature. 1981 Aug 6;292(5823):511–516. doi: 10.1038/292511a0. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., Fraser S. E. Magnetic resonance microscopy of embryonic cell lineages and movements. Science. 1994 Feb 4;263(5147):681–684. doi: 10.1126/science.7508143. [DOI] [PubMed] [Google Scholar]
- Neff A. W., Yokota H., Chung H. M., Wakahara M., Malacinski G. M. Early amphibian (anuran) morphogenesis is sensitive to novel gravitational fields. Dev Biol. 1993 Jan;155(1):270–274. doi: 10.1006/dbio.1993.1024. [DOI] [PubMed] [Google Scholar]
- Pollard E. C. Theoretical studies on living systems in the absence of mechanical stress. J Theor Biol. 1965 Jan;8(1):113–123. doi: 10.1016/0022-5193(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Souza K. A., Black S. D., Wassersug R. J. Amphibian development in the virtual absence of gravity. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1975–1978. doi: 10.1073/pnas.92.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. P., Gerhart J. C. Subcortical rotation in Xenopus eggs: an early step in embryonic axis specification. Dev Biol. 1987 Oct;123(2):526–539. doi: 10.1016/0012-1606(87)90411-8. [DOI] [PubMed] [Google Scholar]
- Weilert MA, Whitaker DL, Maris HJ, Seidel GM. Magnetic Levitation and Noncoalescence of Liquid Helium. Phys Rev Lett. 1996 Dec 2;77(23):4840–4843. doi: 10.1103/PhysRevLett.77.4840. [DOI] [PubMed] [Google Scholar]