Abstract
Background
Flow cytometry (FCM) plays a crucial role in the differential diagnosis of Burkitt lymphoma/leukemia (BL) and B-cell precursor acute lymphoblastic leukemia (BCP-ALL). The presence of surface IgM (sIgM) alone or with light chain restriction indicates a mature blast phenotype (BIV by EGIL) and is usually observed in BL. However, sIgM expression could also be detected in transitional BCP-ALL cases. These similarities in immunophenotype and ambiguous correspondence with other laboratory findings may challenge the correct BL diagnostics.
Methods
We retrospectively reviewed the available data from immunophenotypic, morphological, cytogenetic, and molecular genetic studies of 146 children (85 boys and 61 girls) with a median age of 10 years (range 0–18 years) who were diagnosed with BL and BCP-ALL. The blasts’ immunophenotype was studied by multicolor FCM. The conventional cytogenetic analysis included G-banded karyotyping and fluorescence in situ hybridization (FISH).
Results
In 54 children classified as BIV-ALL according to the EGIL, it was demonstrated that sIgM in a minority of cases can be associated with various types of BCP-ALL. Analysis of the antigen expression profile of 105 patients with verified BL (n = 21) and BCP-ALL (n = 84) showed significant differences in BL and the sIgM(+) vs BCP-ALL immunophenotype. Thus, even in cases of ambiguous sIgM expression, these two diseases could be reliably discriminated by complex immunophenotyping. Moreover, 10 patients (7 boys and 3 girls) with BL leukemic cells did not express sIgM, and they were diagnosed with BL on the basis of other laboratory and clinical signs.
Conclusions
In conclusion, our study shows that BIV subtype is heterogeneous group of leukemia including not only the BL, but also BCP-ALL. In ambiguous cases, only a combination of multiple immunophenotypic, cytomorphologic, and genetic diagnostic technologies can allow the precise discrimination of BL and BCP-ALL and selection of the appropriate treatment scheme.
Electronic supplementary material
The online version of this article (10.1007/s00432-019-03010-1) contains supplementary material, which is available to authorized users.
Keywords: Burkitt lymphoma, BCP-ALL, Flow cytometry, Differential diagnostics
Introduction
Flow cytometric immunophenotyping is one of the key methods for the accurate diagnosis of acute lymphoblastic leukemia (ALL). It is a reproducible and rapid procedure that allows quick identification of the leukemia lineage and thus timely initiation of appropriate treatment. The most common and widely applied immunophenotypic classification of ALL is the European Group for the Immunological Characterization of Leukemias (EGIL) (Bene et al. 1995). Historically, the “mature” BIV subtype defined by surface immunoglobulin μ heavy chain and/or one of light chain expression is considered to be a leukemic manifestation of Burkitt lymphoma (BL). This immunophenotype is commonly associated with the L3 morphology of blast cells according to the FAB classification and rearrangements in the MYC gene (Swerdlow et al. 2017). In most cases, all three of these BL hallmarks coexist. Once the BIV immunophenotype is confirmed, treatment according to the protocols developed for the therapy of mature B-cell lymphomas is to be started immediately. However, expression of Ig heavy or light chains is sometimes detected on cells lacking other signs of mature lymphoma (FAB L3 morphology, MYC rearrangement, and high proliferative activity) (Kansal et al. 2004; Li and Lew 2003; Tsao et al. 2004). At this point, some leading researchers insist on classifying these cases as B-cell precursor ALL (BCP-ALL); therefore, they must be treated as ALL, rather than BL (Dworzak et al. 2017). This argues against the invariable correlation between the “mature” B-cell immunophenotype of blasts and the diagnosis of BL. MYC rearrangements are commonly detected in many types of lymphomas, such as diffuse large cell lymphoma or lymphoma with a plasmablastic morphology (Kleo et al. 2019; Taddesse-Heath et al. 2010). Finally, flow cytometric detection of immunoglobulin light and heavy chains can be hampered by various technical issues. The present study aimed to determine the predictive significance of the flow cytometric detection of surface IgM for differentiation between BL and BCP-ALL.
Patients and methods
We retrospectively reviewed the available data from immunophenotypic, morphological, cytogenetic, and molecular genetic studies of patients who were diagnosed with BL or BCP-ALL from 2010 to 2016. A total of 146 children (85 M and 61 F) with a median age of 10 years (range 0–18 years) were enrolled. Fifty-four patients (40 boys; 14 girls) (median age 8 years; range 0–18 years) with BIV-ALL diagnosed on the basis of sIgM expression were retrieved for evaluation of this subtype heterogeneity. The data from the other 105 patients with verified diagnoses of BL and BCP-ALL, including 55 boys and 50 girls (aged 1–16 years), were used to characterize in depth the immunophenotypic differences between BL and BCP-ALL without considering surface IgM expression. Finally, in 10 patients (7 boys and 3 girls), leukemic cells did not express sIgM, but they were diagnosed with BL because of histopathology data and clinical presentation.
The immunophenotypic analysis of bone marrow cells was performed with 6–8-color flow cytometry in accordance with Moscow–Berlin-group diagnostic standards (Novikova et al. 2018). The panel of monoclonal antibodies (mAb) recognizing antigens CD45, CD19, CD3, CD10, CD34, CD13, CD33, CD117, CD15, CD58, CD38, CD20, CD22, CD79a, CD7, CD5, NG2, µ-chain, κ-chain, λ-chain, TdT, and MPO was applied. Samples were processed according to the manufacturer’s instructions. The Lyse/wash method was applied. At least 30,000 events were acquired per tube using FACS Canto and FACS Canto II flow cytometers (both from Beckton Dickinson, BD, US). Cytometric data were analyzed by FACS Diva 6.1 (BD) and Kaluza 2.1 software (Beckman Coulter, US). The leukemic blasts were identified and gated by the side scatter (SSC) properties and the expression of CD45 and CD19 (Novikova et al. 2018). The cell population was considered positive for the antigen of interest if more than 20% of blasts expressed it on the cell surface or at least 10% did so in the cytoplasm or nucleus. Residual normal cells from the same sample were used as an internal control.
The conventional cytogenetic analysis included G-banded karyotyping and fluorescence in situ hybridization (FISH). Briefly, bone marrow aspirates were cultivated overnight without mitogenic stimulation and G-banding was performed as previously described (den Nijs et al. 1985). Karyotypes were analyzed according to the international cytogenetic nomenclature ICSN 2016 (McGowan-Jordan et al. 2016). MYC gene rearrangements were determined using the Vysis LSI MYC break-apart probe (Abbott Molecular, USA). KMT2A gene rearrangements were identified using the Vysis LSI KMT2A break-apart probe (Abbott Molecular, USA). t(9;11)(9p21.3;q23)/KMT2A-MLLT3 rearrangement was confirmed using the Kreatech ON KMT2A/MLLT3 t(9;11) Dual Color Dual Fusion translocation probe (Leica Microsystems B.V., The Netherlands). The t(1;19)(q23;p13)/TCF3-PBX1 rearrangement was determined using the Vysis LSI TCF3/PBX1 Dual Color Dual Fusion translocation probe (Abbott Molecular, USA).
Statistical analysis of the data sets was performed using XLSTAT-2016. The Yates-corrected Chi-square test was used to compare groups of patients by the number of cases positive for the selected markers. The Mann–Whitney test was applied to compare continuous variables in the two groups.
The study was approved by the local Ethics Committees. Informed consent for the collection and investigation of samples was obtained from patients’ parents or legal guardians.
Results
To evaluate whether IgM detection in bone marrow blasts is essential for diagnosing BL, we divided our study into three consecutive parts. First, we evaluated the heterogeneity of the patient groups with confirmed sIgM expression; second, we compared the immunophenotypes of BL and BCP-ALL; third, we described the discordant diagnostic results for BL and BCP-ALL obtained by different methods.
Heterogeneity of ALL with surface expression of immunoglobulin light and heavy chains
We reviewed the data from immunophenotypic, morphological, cytogenetic, and molecular genetic studies of 54 patients with surface IgM detected on leukemic blasts in bone marrow. Of these patients, 39 were clinically and morphologically diagnosed with BL (median age 11 years), while the remaining 15 cases were classified as the BCP-ALL group (median age 3 years) (Supplementary Table 1).
All patients diagnosed with BL had one of the following MYC rearrangements: t(8;14) (q24;q32) in 28, t(8;22)(q24;q11) in 2, and t(2;8)(p22;q23) in 1 patient. In contrast, none of these translocations were detected in the BCP-ALL group. KMT2A gene rearrangements were found only in patients with BCP-ALL. In the remaining patients in the BCP-ALL group, no specific rearrangements were detected. One patient aged under 12 months with the t(9;11)(p22;q23) had three distinct subpopulations of blast cells: 22% of these blast cells demonstrated the BI variant of ALL, 55% were assigned to the BII subtype, and 23% demonstrated the BIV variant.
After reviewing the morphology of the blast cells, FAB L1/L2 blast morphology was identified in four (10%) of 39 patients diagnosed with BL. In patients with BCP-ALL, this morphological variant was detected in 10 (67%) of 15 children. The FAB L3 morphology was established in 27 patients (51%) with BL and two patients (11%) with BP-ALL (Fig. 1).
Fig. 1.
Rearrangements MYC (blue) and KMT2A (red) on the patients with BIV-ALL (a). Distribution of morphological variants of blast cells according to FAB classification in patients with BIV immunophenotype (b)
The study of immunophenotypic features of bone marrow cells showed a significant difference between the BL and BCP-ALL groups (Table 1, left panel). Different expression of CD20, CD34, CD45, IgM, iIgM, NG2, and CD133 was also observed (Table 1, right panel). NG2, which is expressed generally by microglial cells and acute leukemia blasts with KMT2A abnormalities, was detected in four (25%) of patients with BCP-ALL. CD133, a marker of early hematopoietic progenitor cells, was detected in 30%. Neither marker was ever identified in patients with BL. There was also a prominent difference in the number of samples positive for CD20 and CD34. The expression of CD20, which is a more mature B-cell marker, was mostly observed in patients with BL, whereas a hematopoietic progenitor cell antigen CD34 was more often found in the BCP-ALL group (Table 1, right panel).
Table 1.
Antigens expression by tumor cells in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and Burkitt lymphoma (BL) among EGIL BIV subtype
| Antigens | Number of positive patients (total number of tested patients) | Chi-square p value (number positive patients) | Positive marker expression, % | Mann–Whitney test p value (% positive expression) | ||||
|---|---|---|---|---|---|---|---|---|
| BCP-ALL | BL | BCP-ALL | BL | |||||
| Median | Range | Median | Range | |||||
| CD10 | 14 (15) | 31 (31) | 0.8782 | 99.55 | 9–100 | 98 | 53–100 | 0.418 |
| CD19 | 15 (15) | 39 (39) | 1 | 100 | 95–100 | 100 | 59–100 | 0.134 |
| CD20 | 10 (15) | 38 (39) | 0.5597 | 42.8 | 21–100 | 99.05 | 75–100 | 0.002 |
| CD34 | 7 (15) | 2 (36) | 0.0164 | 100.0 | 32–100 | 49.0 | 20–78 | 0.11 |
| CD38 | 10 (10) | 31 (31) | 1 | 99.0 | 92–100 | 99.0 | 70–100 | 0.757 |
| CD45 | 10 (15) | 39 (39) | 0.5214 | 100 | 96–100 | 100 | 70–100 | 0.469 |
| IgM | 15 (15) | 39 (39) | 1 | 65 | 2.5–95 | 95 | 60–100 | < 0.0001 |
| iIgM | 10 (11) | 25 (28) | 0.9722 | 75 | 15–99 | 97.35 | 84–100 | 0.000 |
| NG2 | 3 (12) | 0 (23) | 0.1054 | 22 | 21–68 | – | – | – |
| CD133 | 3 (10) | 0 (16) | 0.1567 | 57.4 | 51–84 | – | – | – |
| Myeloa | 0 (15) | 1 (34) | 0.5084 | – | – | – | – | – |
aMyeloid markers CD13/CD33/CD117/CD15
Although surface IgM expression was determined in all cases and was the main inclusion criterion for this part of the study, the percentage of IgM-positive cells was higher in BL and accounted for 88.7% versus 55.2% in BCP-ALL patients.
Differences in immunophenotype of BL and BCP-ALL not considering surface IgM expression
We retrospectively compared the antigen expression profile of leukemic blasts in 21 BL and 84 BCP-ALL patients. According to the EGIL classification criteria (Bene et al. 1995), among BCP-ALL patients, 78 were diagnosed with BII, five patients had a BI variant, and one patient had a BIII immunophenotype.
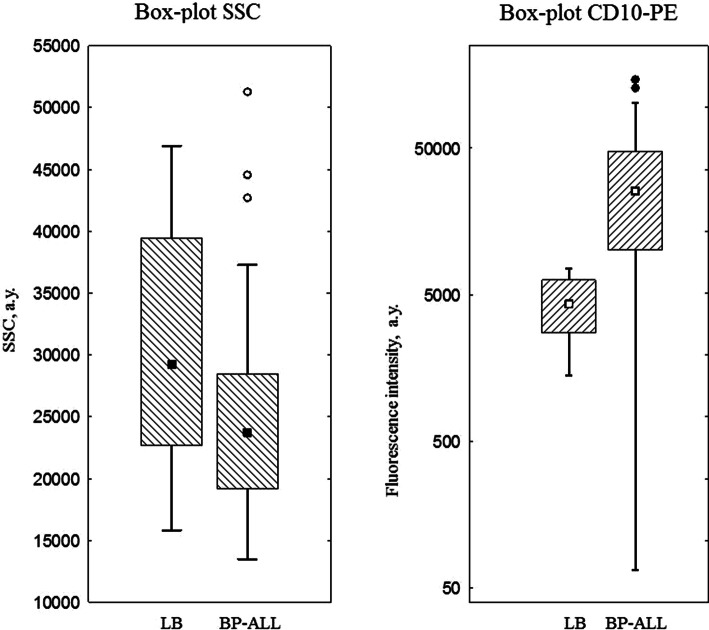
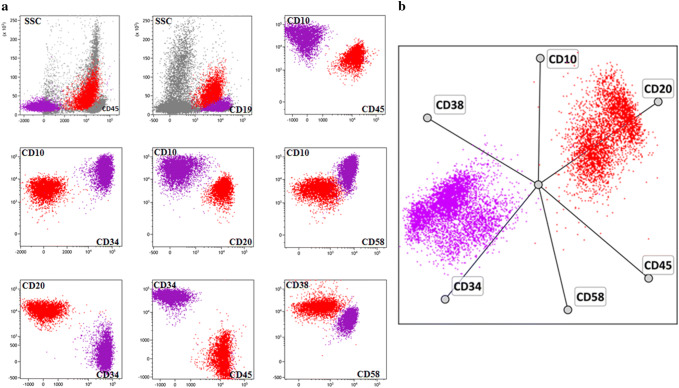
The antigen profiles of blast cells in patients with BL and BCP-ALL were significantly different (Table 2). In addition to the obvious differences in the expression of surface and cytoplasmic light or heavy chains of IgM, the number of patients expressing CD20, CD45, CD34, CD58, and myeloid markers was also variable. Of the 84 patients with BCP-ALL, 47 (56%) displayed coexpression of at least one of the myeloid antigens, while none of the BL patients had any myeloid markers. Blast cells of all the BL patients lacked CD34 and CD58 expression, but were homogeneously positive for CD10, CD38, CD20, and CD45. Although the number of CD10-positive patients between the study group and the comparison group did not show any significant variations, different intensities of CD10 expression were observed (Fig. 2). Furthermore, tumor cells demonstrated different side scatter profiles that approximately represent the level of subcellular compartmentalization (Fig. 2). Dot-plots representing a typical position of tumor cells are shown in Fig. 3.
Table 2.
Antigens expression by tumor cells in ordinary B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and Burkitt lymphoma (BL)
| Antigens | Number of positive patients (total number of tested patients) | % Total number of tested patients | p | ||
|---|---|---|---|---|---|
| BCP-ALL | BL | BCP-ALL | BL | ||
| CD10 | 80 (84) | 21 (21) | 95 | 100 | 0.579 |
| CD19 | 84 (84) | 21 (21) | 100 | 100 | 1 |
| CD20 | 32 (84) | 21 (21) | 38 | 100 | 0.006 |
| CD22 | 65 (66) | 16 (17) | 98 | 94 | 0.873 |
| CD34 | 62 (84) | 0 (21) | 74 | 0 | < 0.0001 |
| CD45 | 66 (84) | 21 (21) | 79 | 100 | 0.033 |
| CD58 | 76 (81) | 0 (7) | 94 | 0 | < 0.0001 |
| IgM | 0 (84) | 21 (21) | 0 | 100 | < 0.0001 |
| κ | 0 (84) | 6 (18) | 0 | 33 | < 0.0001 |
| λ | 0 (84) | 11 (18) | 0 | 61 | < 0.0001 |
| iCD79a | 84 (84) | 21 (21) | 100 | 100 | 0.464 |
| NG2 | 2 (84) | 0 (21) | 2 | 0 | 0.875 |
| Myelo | 47 (84) | 0 (21) | 56 | 0 | 0.003 |
Myelo myeloid markers (CD13/CD33/CD117/CD15), i intracellular marker
Fig. 2.
Differences in expression of CD10 (HI10a-PE) in Burkitt’s lymphoma/leukemia (BL; n = 13) and B-precursor acute lymphoblastic leukemia (BP-ALL; n = 59) (a); Differences in side scatter (SSC) values in Burkitt’s lymphoma/leukemia (LB; n = 21) and B-precursor acute lymphoblastic leukemia (BP-ALL; n = 84) (b)
Fig. 3.
An example of typical distribution of tumor cells on scatter point plots for Burkitt’s lymphoma/leukemia (red) and B-precursor acute lymphoblastic leukemia (purple). Results of immunophenotyping of two patients were combined into a single data set. Both two-dimensional (a) and multidimensional (b) analyses show significant differences between immunophenotypes of tumor blasts. Normal bone marrow cells are shown in gray
Evaluation of the diagnostic efficiency parameters of each marker showed that surface antigen CD58 demonstrated the best accuracy in distinguishing the study group and the comparison group (Table 2).
Discordant laboratory signs in diagnosing Burkitt lymphoma
This part of the study included ten patients who had an immunophenotype of BCP-ALL, but were finally diagnosed with BL between 2010 and 2016. Details of the immunophenotypic, morphological, and cytogenetic examination of patients are presented in Table 3. There were two cases without classical rearrangements of the MYC gene. In addition, in one patient, translocations t(8;14)(q24;q32), t(14;18)(q32;q21) were found simultaneously in the absence of surface IgM expression. The L2 cytological variant was determined in three patients, which contradicted the presence of MYC gene changes typical for LB. In one case, undifferentiated blasts were described in the presence surface IgM and without rearrangements of the MYC gene and extramedullary lesions.
Table 3.
Clinical and laboratory characteristics of ten cases of BL with aberrant phenotype
| No. | Sex | Age | Extramedullary lesions | Immunophenotype | B-cell lineage antigens | Additional antigens | Morphology | Cytogenetic | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM | CD19 | CD20 | CD10 | CD34 | CD38 | CD45 | |||||||
| 1 | f | 13 | Ovary | B-IV | + | + | + | + | − | NA | + | Undifferentiated blast cells | MYC rearrangement not detected |
| 2 | m | 7 | Yes, localization is unknown | B-IV | + | + | + | + | − | + | + | L3 | MYC rearrangement not detected |
| 3 | m | 4 | NA | B-IV | + | + | + | + | NA | NA | + | L2 | t(8;14)(q24;q32) |
| 4 | m | 1 | Skull bones | B-III | − | + | − | + | − | NA | + | L3 | t(8;14)(q24;q32) |
| 5 | m | 14 | Mediastinum | B-II | − | + | + | + | − | + | + | L3 | t(8;14)(q24;q32) |
| 6 | m | 10 | Pancreas | B-II | − | + | + | + | − | NA | + | NA | t(8;14)(q24;q32) |
| 7 | m | 8 | Intraocular | B-II | − | + | + | + | − | NA | + | L2 | t(8;14)(q24;q32), t(14;18)(q32;q21) |
| 8 | m | 13 | Intestine, liver, kidney | B-II | − | + | + | + | − | + | + | L3 | t(8;14)(q24;q32) |
| 9 | m | 16 | Ileum | B-II | − | + | + | + | − | + | + | L3 | t(8;22)(q24;q11) |
| 10 | f | 15 | NA | B-II | − | + | + | + | − | + | + | L3 | t(8;14)(q24;q32) |
(+) positive, (−) negative, f female, m male, NA not available
In one patient, in contrast to the classical BL immunophenotype, no sIgM or CD20 expression was found, whereas a λ chain was expressed intracellularly (Fig. 4). An MRI scan revealed a diffuse calvarial lesion. Treatment according to the ALL-MB-2008 protocol was started (Popov et al. 2019). However, detection of the t(8;14)(q24;q32) along with the L3 blast morphology led to reconsideration of the diagnosis. The treatment protocol was changed to the BFM-NHL-90 plus rituximab (Samochatova et al. 2014).
Fig. 4.
Immunophenotype of blasts of patient with BL. No sIgM and CD20 expression was found, whereas λ chain expressed intracellularly (tumor cells are painted red, while residual B-lymphocytes are painted blue, i—intracellular expression)
Discussion
Clear and prompt separation of BL from BCP-ALL is important, because erroneous allocation of a patient to the wrong treatment protocol makes a relapse-free course and cure almost impossible. Analysis of the patient group with a mature immunophenotype revealed heterogeneous features. We found that 28% of patients with unequivocal sIgM expression lack BL-specific rearrangements of the MYC gene as well as L3 blast morphology. They had either BCP-ALL-associated genetic rearrangements or cell morphology that did not meet the FAB criteria of L3. Two patients with L1/L2 FAB morphology displayed MYC rearrangements and were diagnosed with BL, showing that morphology alone is not absolutely sensitive and specific. Some of the previously published clinical cases reported contradictory data about key laboratory findings (Kelemen et al. 2010). The authors also reported search strategies and treatment outcomes (Li and Lew 2003; Tsao et al. 2004; Frater et al. 2004; Blin et al. 2008). Some reports demonstrated a few patients with KMT2A abnormalities in the tumor clone, mainly t(9;11)(p22;q23) (Kim et al. 2014; Krivtsov and Armstrong 2007). Such patients received conventional ALL treatment, and appropriate results were achieved. Most likely, similar cases could be considered BCP-ALL with aberrant expression of surface immunoglobulin and probably should be treated according to the protocols for ALL. Among these cases, there have been a small number of patients with KMT2A rearrangements, mainly the t(9;11)(p22;q23) (Kim et al. 2014; Blin et al. 2008). One study (Smith et al. 2015) presented the case of BCP-ALL from mature cells with L3 morphology and a t(11;15)(q23;q14) rearrangement without KMT2A involvement. Thus, the group of patients with surface IgM expression is heterogeneous; it includes not only BL but also various cases of IgM-positive BCP-ALL with or without KMT2A rearrangements.
In this work, we demonstrated that BL differs significantly from IgM-positive BCP-ALL not only by the expression of IgM but also by other surface markers. Therefore, even with a discordant or negative result of surface IgM detection, differential diagnosis of these two tumors can be suggested with a complex analysis of the immunophenotype. After reviewing immunophenotypic data of verified BL and BCP-ALL, a few peculiarities of the BL immunophenotype were found. Mentioned differences could help to interpret cytometry results in the differential diagnosis of B-lineage ALL. It should be borne in mind that BL tumor cells are most often characterized by the absence of myeloid markers and CD34. In the case of detection of such a phenotype, it is necessary to pay close attention to the level of side scatter and the proportion of CD20-positive cells, which is significantly higher in BL than in BCP-ALL. In addition, the CD58 antigen, which is detectable in various cells of hematopoietic and nonhematopoietic origin, deserves a special attention. Previously, the expression of CD58 has been frequently shown in almost all cases of BCP-ALL. In contrast, CD58 expression in BL is rarely reported (Veltroni et al. 2003; Lee et al. 2005). Consistent with the study of M. Veltroni et al. (2003), our data showed the absence of CD58 in all cases of BL. In addition, its expression in BCP-ALL was often high and was absent only in 5% of cases. However, the expression of this antigen was investigated only in seven patients with BL; thus, this is to be further confirmed.
DNA cytometry may provide additional diagnostic information. Blasts in BL have extremely high proliferative activity; therefore, it would be rational to consider measuring the proportion of cells in the mitotic phase of the cell cycle by DNA cytometry. This method could help in the differential diagnosis of BL and BCP-ALL in doubtful cases. One small sample study showed the difference between the morphological types of L1–L2 and L3 using DNA cytometry (Lichtman and Andreeff 1992). Analysis of the fine-needle cytological aspirates of lymph nodes demonstrated that BL has a greater number of cells in S-phase compared to other non-Hodgkin’s lymphomas (Pinto et al. 2003). However, due to the ambiguity of the morphology findings in determining the final diagnosis, this approach is not widely used.
In the updated WHO classification published in 2016 (Cazzola 2016), it was noted that there are no absolute diagnostic criteria for BL. Even the MYC rearrangements are not completely specific for BL; they can be found in other hematologic diseases. The same applies to both morphological and immunophenotypic findings. The most recent version of the WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues added the Bcl2 and Bcl6 markers to the immunophenotypic panel. However, in cases with a progenitor phenotype of blasts, these markers failed to clarify the diagnosis (Swerdlow et al. 2017). Thus, the group of patients with detected surface IgM on blast cells is fairly heterogeneous and includes not only BL but also rare cases of BCP-ALL, including those with rearrangements of the KMT2A gene. Special attention in immunophenotypic diagnosis should be paid not only to the presence of IgM on the surface of blast cells but also to other markers that distinguish blasts in lymphomas and ALL. Moreover, BL may not show a mature immunophenotype or L3 morphology, and, although rarely, may lack MYC rearrangements.
In conclusion, our study shows that BIV subtype is heterogeneous group of leukemia including not only the BL, but also BCP-ALL. Moreover, BL cells in BM sometimes lack sIgM expression or have other controversial laboratory findings. Nevertheless, BL immunophenotype, even without taking into account the expression of surface IgM, differs significantly from other types of BCP-ALL. Thus, complex analysis of immunophenotypic, morphological, and molecular genetic findings can provide reliable data to help in the differential diagnosis of BL from BCP-ALL and choose the correct treatment strategy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Authors declare no relevant conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bene M, Castoldi G, Knapp W et al (1995) Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 9(10):1783–1786 [PubMed] [Google Scholar]
- Blin N, Méchinaud F, Talmant P, Garand R, Boutard P, Dastugue N et al (2008) Mature B-cell lymphoblastic leukemia with MLL rearrangement: an uncommon and distinct subset of childhood acute leukemia. Leukemia 22:1056–1059. 10.1038/sj.leu.2404992 [DOI] [PubMed] [Google Scholar]
- Cazzola M (2016) Introduction to a review series: the 2016 revision of the WHO classification of tumors of hematopoietic and lymphoid tissues. Blood 127(20):2361–2364. 10.1182/blood-2016-03-657379(Epub 11 Apr 2016) [DOI] [PubMed] [Google Scholar]
- den Nijs JI, Gonggrijp HS, Augustinus E, Leeksma CH (1985) Hot bands: a simple G-banding method for leukemic metaphases. Cancer Genet Cytogenet 15(3–4):373–374 [DOI] [PubMed] [Google Scholar]
- Dworzak MN, Buldini B, Gaipa G et al (2017) AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of pediatric acute lymphoblastic leukemia. Cytom B Clin Cytom 94(1):82–93. 10.1002/cyto.b.21518 [DOI] [PubMed] [Google Scholar]
- Frater JL, Batanian JR, O’Connor DM, Grosso LE (2004) Lymphoblastic leukemia with a mature B-cell phenotype in infancy. J Pediatr Hematol Oncol 26:672–677. 10.1097/01.mph.0000142493.56793.cb [PubMed] [Google Scholar]
- Kansal R, Deeb G, Barcos M et al (2004) Precursor B lymphoblastic leukemia with surface light chain immunoglobulin restriction: a report of 15 patients. Am J Clin Pathol 121:512–525. 10.1309/WTXC-Q5NR-ACVX-TYBY [DOI] [PubMed] [Google Scholar]
- Kelemen K, Braziel RM, Gatter K, Bakke TC, Olson S, Fan G (2010) Immunophenotypic variations of Burkitt lymphoma. Am J Clin Pathol 134:127–138. 10.1309/AJCP93LJPTRQPDKR [DOI] [PubMed] [Google Scholar]
- Kim B, Lee ST, Kim HJ, Lee SH, Yoo KH, Koo HH (2014) Acute lymphoblastic leukemia with mature B-cell phenotype and t(9;11;11)(p22;q23;p11.2): a case study and literature review. Ann Lab Med 34:166–169. 10.3343/alm.2014.34.2.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleo K, Dimitrova L, Oker E, Tomaszewski N, Berg E, Taruttis F, Engelmann JC, Schwarzfischer P, Reinders J, Spang R, Gronwald W, Oefner PJ, Hummel M (2019) Identification of ADGRE5 as discriminating MYC target between Burkitt lymphoma and diffuse large B-cell lymphoma. BMC Cancer 19(1):322–333. 10.1186/s12885-019-5537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA (2007) MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer 7:823–833. 10.1038/nrc2253 [DOI] [PubMed] [Google Scholar]
- Lee RV, Braylan RC, Rimsza LM (2005) CD58 expression decreases as nonmalignant B cells mature in bone marrow and is frequently overexpressed in adult and pediatric precursor B-cell acute lymphoblastic leukemia. Am J Clin Pathol 123(1):119–124. 10.1309/x5vv6fkjq6mublpx [DOI] [PubMed] [Google Scholar]
- Li S, Lew G (2003) Is B-lymphoblastic leukemia/lymphoma or Burkitt leukemia/lymphoma? Arch Pathol Lab Med 127:1340–1344. 10.1043/1543-2165(2003)127%3c1340:IBALLW%3e2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lichtman SM, Andreeff M (1992) Flow cytometric analysis of cytokinetics of L3-acute lymphoblastic leukemia/lymphoma. Leuk Res 16(9):853–859 [DOI] [PubMed] [Google Scholar]
- McGowan-Jordan J, Simons A, Schmid M (eds) (2016) ISCN 2016: an international system for human cytogenomic nomenclature (2016). Reprint of: Cytogenetic and Genome Research 149(1–2). Karger Publishers, Basel
- Novikova IA, Verzhbitskaya TY, Movchan LV, Tsaur GA, Belevtsev MV, Popov AM (2018) Russian-belarusian multicenter group standard guidelines for childhood acute lymphoblastic leukemia flow cytometric diagnostics. Oncohematology 13(1):73–82. 10.17650/1818-8346-2018-13-1-73-82 [Google Scholar]
- Pinto AE, Cabeçadas J, Nóbrega SD, Mendonça E (2003) Flow cytometric S-phase fraction as a complementary biological parameter for the cytological grading of non-Hodgkin’s lymphoma. Diagn Cytopathol 29(4):194–199. 10.1002/dc.10298 [DOI] [PubMed] [Google Scholar]
- Popov A, Henze G, Verzhbitskaya T, Roumiantseva J, Lagoyko S, Khlebnikova O, Streneva O, Bidanov O, Tsaur G, Inaba H, Karachunskiy A, Fechina L (2019) Absolute count of leukemic blasts in cerebrospinal fluid as detected by flow cytometry is a relevant prognostic factor in children with acute lymphoblastic leukemia. J Cancer Res Clin Oncol 145(5):1331–1339. 10.1007/s00432-019-02886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochatova E, Maschan A, Shelikhova L, Myakova N, Belogurova M, Khlebnikova O, Shamardina A, Ryskal O, Roumiantseva J, Konovalov D, Dubrovina M, Rumyantsev A (2014) Therapy of advanced-stage mature B-cell lymphoma and leukemia in children and adolescents with rituximab and reduced intensity induction chemotherapy (B-NHL 2004 M protocol): the results of a multicenter study. J Pediatr Hematol/Oncol 36(5):395–401. 10.1097/MPH.0b013e31829d4900 [DOI] [PubMed] [Google Scholar]
- Smith MC, Kressin MK, Crawford E, Wang XJ, Kim AS (2015) B lymphoblastic leukemia with a novel t (11; 15) (q23; q15) and the Burkittoid morphologic and immunophenotypic findings in a 9-year-old boy. Lab Med Fall 46:320–326. 10.1309/LM0BOC84GSQGHYKD [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R (2017) WHO classification of haematopoietic and lymphoid tissues, Revised 4 edn. International Agency for Research on Cancer, Lyon [Google Scholar]
- Taddesse-Heath L, Meloni-Ehrig A, Scheerle J, Kelly JC, Jaffe ES (2010) Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features. Mod Pathol. 23:991–999. 10.1038/modpathol.2010.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao L, Draoua HY, Osunkwo I et al (2004) Mature B-cell acute lymphoblastic leukemia with t(9; 11) translocation: a distinct subset of B-cell acute lymphoblastic leukemia. Mod Pathol 17:832–839. 10.1038/modpathol.3800128 [DOI] [PubMed] [Google Scholar]
- Veltroni M, De Zen L, Sanzari MC, Maglia O, Dworzak MN, Ratei R, Biondi A, Basso G, Gaipa G, I-BFM-ALL-FCM-MRD-Study Group (2003) Expression of CD58 in normal cells, regenerating and leukemic bone marrow B lymphocytic leukemia. Haematologica 88(11):1245–1252 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.