Abstract
Purpose
Nodal positive lung adenocarcinoma includes wide range of survival. Several methods for the classification of nodal-positive lung cancer have been proposed. However, classification considering the impact of targetable genetic variants are lacking. The possibility of genetic variants for the better stratification of nodal positive lung adenocarcinoma was estimated.
Methods
Mutations of 36 genes between primary sites and metastatic lymph nodes (LNs) were compared using next-generation sequencing. Subsequently, mutations in EGFR and BRAF, rearrangements in ALK and ROS1 were evaluated in 69 resected pN1–2M0 adenocarcinoma cases. Recurrence-free survival (RFS), post-recurrence survival (PRS), and overall survival (OS) were evaluated with respect to targetable variants and tyrosine kinase inhibitor (TKI) therapy after recurrence.
Results
About 90% of variants were shared and allele frequencies were similar between primary and metastatic sites. In 69 pN1–2M0 cases, EGFR/ALK were positive in primary sites of 39 cases and same EGFR/ALK variants were confirmed in metastatic LNs of 96.7% tissue-available cases. Multivariate analyses indicated positive EGFR/ALK status was associated with worse RFS (HR 2.366; 95% CI 1.244–4.500; P = 0.009), and PRS was prolonged in cases receiving TKI therapy (no post-recurrence TKI therapies, HR 3.740; 95% CI 1.449–9.650; P = 0.006). OS did not differ with respect to targetable variants or TKI therapy.
Conclusions
Cases harbouring targetable genetic variants had a higher risk of recurrence, but PRS was prolonged by TKI therapy. Classification according to the targetable genetic status provides a basis for predicting recurrence and determining treatment strategies after recurrence.
Electronic supplementary material
The online version of this article (10.1007/s00432-019-02978-0) contains supplementary material, which is available to authorized users.
Keywords: Lung adenocarcinoma, Lymph node metastasis, NGS, Recurrence, EGFR, ALK
Introduction
Lung cancer with metastatic lymph nodes (LNs) is an advanced status with variation in prognosis. Nodal-positive cases have a high risk of recurrence, even after complete resection. Recurrence is a crucial issue for prognosis after resection, and unresectable recurrent cases harbouring targetable genetic variants are good candidates for targeted therapies. Although several further classification methods have been proposed for nodal-positive cases by analysing the metastatic lymph node (LN) status, methods based on targetable genetic variants are lacking. Additionally, the prognostic impact of genetic aberrations in metastatic LNs is unclear. This study aimed to compare the status of targetable genetic variants at primary sites and metastatic LN of lung adenocarcinoma and to explore classification methodology for nodal-positive lung adenocarcinoma based on genetic status and prognostic value.
Materials and methods
Study design
Nodal-positive lung adenocarcinoma cases resected between 2005 and 2016 at Hiroshima University Hospital (Hiroshima, Japan) were retrospectively reviewed. To estimate the spread of genetic variation in nodal metastatic cases, genetic heterogeneity between primary sites and metastatic LNs was first evaluated by next-generation sequencing (NGS). Two pairs of fresh frozen tissues from primary sites and corresponding metastatic LNs were used. Subsequently, mutations in EGFR and BRAF and rearrangements in ALK and ROS1, which are current targetable variants, were detected in primary sites of pN1–2 cases by clinically available methods. In EGFR/ALK/BRAF/ROS1-positive cases, the same targetable variants were evaluated in metastatic LNs. The prognostic impact of targetable variants on pN1–2 patients was evaluated based on recurrence-free survival (RFS), post-recurrence survival (PRS), and overall survival (OS).
pN1–2 cases after neoadjuvant chemotherapy/chemoradiotherapy, proven to be incomplete resection (R1–2), pN3, or distant metastasis were excluded. All included cases were pathologically diagnosed according to the classification system of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) (Travis et al. 2011) or 2015 WHO classification (Travis et al. 2015) by two pathologists (Y. T. and K. K.). TNM stage was determined according to the IASLC TNM classification system (eighth edition) (Rami-Porta et al. 2018). This study, including the utilisation of surgical specimens and collection and analysis of clinicopathological data, was approved by the institutional review board at Hiroshima University Hospital (E-1231).
Next-generation sequencing for comprehensive genetic analysis
For NGS, fresh frozen tissues obtained by surgical resection were used. DNA was extracted using the QIAamp DNA Micro Kit (56403; QIAGEN GmbH, Hilden, Germany). Extracted DNA was prepared for NGS using HaloPlex HS (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s instructions. MiSeq (Illumina Inc., San Diego, CA, USA) was utilized for NGS as previously described (Ito et al. 2017). Sequence reads were processed and mapped to a human genome reference sequence (hg19) using SureCall 3.5.1.46 (Agilent Technologies). The evaluated genes and coverage for each targeted region are shown in Supplemental Table 1. Our custom panel was designed to cover 36 genes with a median coverage of 99.57% (80.89–100%) (Ito et al. 2017). If a mutation met at least one of the following criteria, it was excluded from the analysis as false positive detection: allele frequency < 0.05, number of variant alleles < 10, filtered read depth < 100, or variants defined as benign or likely benign using the default settings of SureCall.
Detection of EGFR/ALK/BRAF/ROS1 variants at primary sites and metastatic LNs
The EGFR mutation status in Exon 18, 19, and 21 was evaluated by the peptide nucleic acid-locked nucleic acid (PNA–LNA) polymerase chain reaction (PCR) clamp method by an institutional laboratory or external estimation body as described previously (Nagai et al. 2005; Ito et al. 2018). BRAF mutation (V600E) was evaluated by LNA PCR in the institutional laboratory. The conditions for LNA PCR and sequence of primers and probes are described in Supplemental Table 2. DNA was extracted from frozen sections as described above or from formalin-fixed paraffin-embedded (FFPE) tissues using a QIAamp DNA FFPE Tissue Kit (56404; QIAGEN GmbH).
Rearrangements in ALK and ROS1 were evaluated according to the College of American Pathologists (CAP)/IASLC/Association for Molecular Pathology (AMP) guideline (Kalemkerian et al. 2018). Immunohistochemistry (IHC) was regarded as an equivalent alternative to fluorescence in situ hybridization (FISH) for ALK rearrangement testing (Kalemkerian et al. 2018). IHC was conducted at the Pathological Department of Hiroshima University Hospital using commercial antibody (727071; NICHIREI BIOSCIENCES INC., Tokyo, Japan) and FISH was performed using the Vysis ALK Break Apart FISH Probe Kit (6N38-21; Abbott JAPAN, Tokyo, Japan) by an external examining body. ROS1 rearrangements were screened by IHC using a commercial antibody (3287; Cell Signaling Technology, Danvers, MA, USA) at an institutional laboratory. Positive IHC results for ROS1 were confirmed by cytomolecular methods using a commercial kit (A163; RIKEN GENESIS CO., Ltd., Tokyo, Japan) by an external examination body. For the detection of EGFR/ALK/ROS1 variants, the same external examination body (LSI Medience Corp., Tokyo, Japan) was utilized.
Statistical analyses
RFS was calculated from the day of operation to the day when recurrence was detected radiologically. PRS was calculated from the day of recurrence to the day of death from any cause. OS was calculated from the day of operation to the day of death from any cause. To compare continuous numerical variables, the Mann–Whitney U test was used. For frequencies, significance was evaluated using the Chi squared, Yates, or Fisher’s exact probability test. RFS, PRS, and OS were estimated using the Kaplan–Meier method and compared using log-rank tests. Univariate and multivariate analyses were conducted by a Cox proportional hazards model with a backward stepwise procedure. Probability values were derived from two-tailed tests and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA) and StatMate V (ATMS Co., Ltd., Tokyo, Japan).
Results
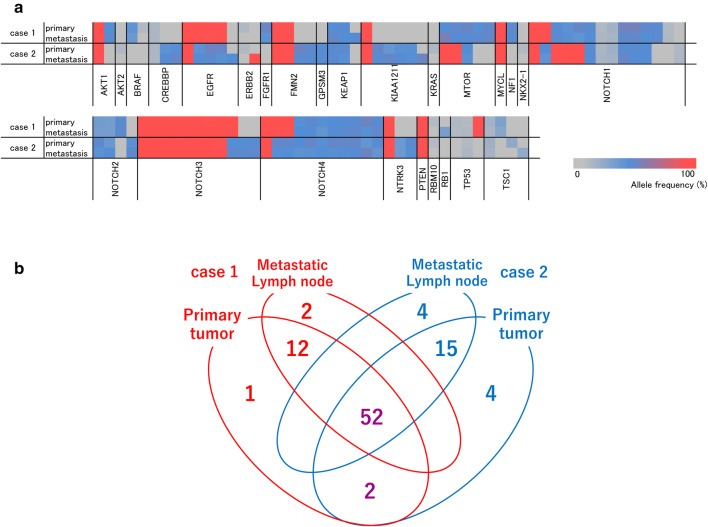
For NGS analyses of primary sites and metastatic LNs, the median read depth and percentage of target regions coverage were 416 (106–1238) and 92.39% (92.13–92.73%), respectively. The genetic variants and allele frequencies were similar between primary sites and metastatic LNs. A heat map of genetic variants showed a symmetric pattern between primary sites and corresponding metastatic LNs in the two cases (Fig. 1a). Moreover, as shown in a Venn diagram, 92.8% (64/69) and 87.0% (67/77) of variants were shared between primary and metastatic sites in case 1 and case 2, respectively (Fig. 1b).
Fig. 1.
NGS-based comparative analysis of mutation type and allele frequency between primary sites and metastatic LNs in two cases. a Heat map showing the allele frequency of each detected variant. b Venn diagram indicating the number of detected genetic variants in primary and/or metastatic LN
A total of 69 pN1–2 adenocarcinoma cases were enrolled and patient characteristics are shown in Table 1. All 69 cases were resected with curative intensity after clinical staging using computed tomography (CT), positron-emission tomography (PET)-CT, and brain magnetic resonance imaging (MRI). As targetable variants, EGFR mutations were confirmed in 35 out of 69 pN1–2 cases. ALK rearrangements were positive in 4 cases (2 cases were positive by IHC and 2 cases were positive by FISH). BRAF mutations and ROS1 rearrangements were not detected (3 cases were checked by the cytomolecular method for ROS1 rearrangement after IHC screening). The median follow-up term was 43.5 (3.75–128.2) months. Forty-seven cases relapsed and 27 cases received tyrosine kinase inhibitor (TKI) therapy after recurrence (gefitinib in 15 patients, erlotinib in 9 patients, afatinib in 8 patients, and alectinib in 1 patient).
Table 1.
Clinicopathological characteristics of pN1–2 adenocarcinoma cases (N = 69)
| Clinicopathological characteristic | Cases (N = 69) |
|---|---|
| Age, years | |
| Median (range) | 68 (45–89) |
| Interquartile range | 13.75 |
| Sex, N (%) | |
| Male | 44 (63.8) |
| Female | 25 (36.2) |
| Smoking status, N (%) | |
| Current or ex-smoker | 41 (59.4) |
| Never smoker | 28 (40.6) |
| Surgical procedure, N (%) | |
| Lobectomy | 60 (87.0) |
| Segmentectomy | 8 (11.6) |
| Wedge resection | 1 (1.5) |
| Predominant subtype, N (%) | |
| Lepidic | 4 (5.8) |
| Acinar | 6 (8.7) |
| Papillary | 42 (60.9) |
| Micropapillary | 3 (4.3) |
| Solid | 12 (17.4) |
| IMA | 2 (2.9) |
| Pathological node descriptor, N (%) | |
| N1a | 18 (26.1) |
| N1b | 7 (10.1) |
| N2a1 | 14 (20.3) |
| N2a2 | 7 (10.1) |
| N2b | 23 (33.3) |
| Pathological stage, N (%) | |
| IIB | 22 (31.9) |
| IIIA | 43 (62.3) |
| IIIB | 4 (5.8) |
| Number of metastatic LN | |
| Median (range) | 2 (1–35) |
| Interquartile range | 6 |
| Ratio of metastatic LN among resected LN, % | |
| Median (range) | 20 (3.3–100) |
| Interquartile range | 32 |
| Classification by nodal zone, N (%) | |
| N1a | 18 (26.1) |
| N1b | 7 (10.1) |
| N2a | 30 (43.5) |
| N2b | 14 (20.3) |
| Adjuvant platinum doublet chemotherapy, N (%) | |
| Y | 41 (59.4) |
| N | 28 (40.6) |
| Targetable variant status, N (%) | |
| EGFR mutation | 35 (50.7) |
| ALK rearrangement | 4 (5.8) |
| EGFR/ALK/BRAF/ROS1 negative | 30 (43.5) |
| Pleural invasion, N (%) | |
| Y | 25 (36.2) |
| N | 44 (63.8) |
| Lymphatic invasion, N (%) | |
| Y | 47 (68.1) |
| N | 22 (31.9) |
| Vascular invasion, N (%) | |
| Y | 41 (59.4) |
| N | 28 (40.6) |
| Recurrence, N (%) | |
| Y | 47 (68.1) |
| N | 22 (31.9) |
| TKI therapy after recurrence, N (%) | |
| Y | 27 (57.4) |
| N | 20 (42.6) |
IMA invasive mucinous adenocarcinoma, LN lymph node, N no, TKI tyrosine kinase inhibitor, Y yes
Among 39 EGFR/ALK-positive pN1–2 cases, LN tissues were available for 30 cases and the same EGFR/ALK-positive status was confirmed in 29 cases (concordance ratio 96.7%).
RFS and OS were evaluated in EGFR/ALK-positive and EGFR/ALK-negative cases. PRS and OS were evaluated in patients who received TKI therapy after recurrence. Genetic results and populations for prognostic comparisons are summarised in Fig. 2.
Fig. 2.
Summary of genetic variants and populations for prognostic comparison
The median RFS were 16.9 and 20.5 months for EGFR/ALK-positive and -negative cases, respectively [hazard ratio (HR) 1.80, 95% confidence interval (CI) 1.02–3.20; P = 0.043]. The median PRS were 34.5 months and 18.7 months for cases with and without TKI therapy, respectively (HR 0.44, 95% CI 0.14–0.91; P = 0.032). There was no significant difference in OS between EGFR/ALK-positive and -negative cases (median OS: 46.3 months vs. 38.5 months, respectively, HR 0.99, 95% CI 0.45–2.20; P = 0.99) or with and without TKI therapies cases (median OS: 49.3 months vs. 38.5 months, respectively, HR 0.65, 95% CI 0.27–1.46; P = 0.28) (Fig. 3).
Fig. 3.
RFS, PRS, and OS curves based on EGFR mutation/ALK rearrangement status or TKI treatment. a RFS curves according to EGFR/ALK status. b PRS curves according to post-recurrence TKI therapies. c OS curves according to EGFR/ALK status. d OS curves according to post-recurrence TKI therapies
Univariate analyses revealed that the ratio of metastatic LNs and EGFR/ALK-positive status were significantly associated with poorer RFS, and no TKI therapy after recurrence was associated with shorter PRS. Multivariate analysis revealed that male sex, no adjuvant platinum doublet chemotherapy, ratio of metastatic LNs, and EGFR/ALK-positive status were predictive factors for worse RFS (for male sex, no adjuvant platinum doublet chemotherapy, ratio of metastatic LN, and positive EGFR/ALK status, HR 2.276, 1.912, 1.012, and 2.366; 95% CI 1.141–4.542, 1.042–3.511, 1.001–1.024, and 1.244–4.500; P = 0.020, 0.037, 0.033, and 0.009, respectively) (Table 2). N2 metastatic status and no TKI therapies after recurrence were predictive factors for shorter PRS (for N2 status and no post-recurrence TKI therapies, HR 3.652 and 3.740; 95% CI 1.022–13.047 and 1.449–9.650; P = 0.046 and 0.006, respectively) (Table 3).
Table 2.
Univariate and multivariate analyses of RFS
| Prognostic factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.021 (0.995–1.048) | 0.115 | 1.017 (0.982–1.054) | 0.341 |
| Male sex | 1.435 (0.786–2.618) | 0.239 | 2.276 (1.141–4.542) | 0.020* |
| Sublobar resection | 1.165 (0.519–2.614) | 0.711 | 0.800 (0.344–1.860) | 0.604 |
| No adjuvant platinum doublet therapy | 1.417 (0.802–2.503) | 0.230 | 1.912 (1.042–3.511) | 0.037* |
| MP/solid/IMA predominance | 0.789 (0.391–1.589) | 0.506 | 0.986 (0.401–2.425) | 0.975 |
| Pleural invasion | 1.408 (0.801–2.476) | 0.234 | 1.167 (0.630–2.162) | 0.624 |
| Lymphatic invasion | 1.129 (0.620–2.056) | 0.691 | 1.089 (0.515–2.303) | 0.824 |
| Vascular invasion | 1.284 (0.715–2.306) | 0.402 | 1.180 (0.569–2.450) | 0.657 |
| Metastasis on N2 station | 1.576 (0.844–2.946) | 0.154 | 1.131 (0.527–2.427) | 0.752 |
| Number of metastatic LNs | 1.029 (0.985–1.074) | 0.199 | 0.991 (0.930–1.057) | 0.783 |
| Ratio of metastatic LNs | 1.013 (1.002–1.024) | 0.017 | 1.012 (1.001–1.024) | 0.033* |
| Metastasis on N2b zone | 1.837 (0.952–3.542) | 0.070 | 1.449 (0.706–2.976) | 0.312 |
| Positive EGFR/ALK | 1.850 (1.009–3.393) | 0.047 | 2.366 (1.244–4.500) | 0.009* |
IMA invasive mucinous adenocarcinoma, LNs lymph nodes, MP micropapillary
*P < 0.05
Table 3.
Univariate and multivariate analyses of PRS
| Prognostic factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.040 (0.997–1.086) | 0.069 | 1.032 (0.988–1.077) | 0.156 |
| Male sex | 1.016 (0.440–2.347) | 0.971 | 1.044 (0.994–1.097) | 0.084 |
| Sublobar resection | 0.649 (0.189–2.230) | 0.492 | 0.646 (0.176–2.367) | 0.510 |
| MP/solid/IMA predominance | 1.180 (0.338–4.119) | 0.796 | 0.604 (0.161–2.261) | 0.454 |
| Pleural invasion | 0.848 (0.379–1.898) | 0.688 | 0.549 (0.214–1.411) | 0.213 |
| Lymphatic invasion | 2.131 (0.840–5.407) | 0.111 | 1.778 (0.686–4.609) | 0.237 |
| Vascular invasion | 1.676 (0.704–3.991) | 0.244 | 1.315 (0.462–3.744) | 0.608 |
| Metastasis on N2 station | 2.211 (0.654–7.472) | 0.202 | 3.652 (1.022–13.047) | 0.046* |
| Number of metastatic LNs | 1.023 (0.959–1.091) | 0.488 | 1.007 (0.908–1.116) | 0.895 |
| Ratio of metastatic LNs | 1.006 (0.991–1.021) | 0.422 | 1.004 (0.984–1.025) | 0.698 |
| Metastasis on N2b zone | 1.303 (0.558–3.042) | 0.541 | 0.708 (0.266–1.883) | 0.489 |
| Positive EGFR/ALK | 0.483 (0.201–1.162) | 0.104 | 0.852 (0.149–4.855) | 0.857 |
| No post-recurrence TKI therapy | 2.551 (1.056–6.162) | 0.037 | 3.740 (1.449–9.650) | 0.006* |
IMA invasive mucinous adenocarcinoma, LNs lymph nodes, MP micropapillary, TKI tyrosine kinase inhibitor
*P < 0.05
Discussion
Lung cancer with metastatic LN is no longer early-stage. Nodal-positive cases are conventionally classified as N1, N2, or N3 according to the location of the metastatic LN. Nodal-positive cases vary with respect to prognosis; new classification methodologies have been proposed (Wei et al. 2011; Jonnalagadda et al. 2011; Saji et al. 2011; Wisnivesky et al. 2011; Rusch et al. 2007, 2009) and their usefulness has been compared (Ito et al. 2013; Lee et al. 2016). Nodal-positive cases have a high risk of recurrence even after complete resection, and PRS is prolonged by EGFR–TKI treatment (Takenaka et al. 2015; Jeon et al. 2015; Shimada et al. 2013). Nevertheless, a classification that considers the post-recurrence course of treatment has not been proposed. We focused on the therapeutic course after recurrence and propose a classification method for nodal-positive cases based on targetable genetic features.
Reported concordances of genetic status between primary and metastatic sites vary according number of invested genes or utilized methodologies. Several studies have compared the genetic status of primary and metastatic sites, focusing on one to three genes, and have reported a wide range of concordance ratios of 26.7–100% (Kalikaki et al. 2008; Park et al. 2009; Cortot et al. 2010; Schmid et al. 2009; Matsumoto et al. 2006). A well-designed study (Yatabe et al. 2011) and another study using an NGS approach (Vignot et al. 2013) concluded that genetic discordance between primary and metastatic sites is rare. Xie et al. also used NGS and showed that driver gene mutations correspond completely (100%) between primary sites and metastatic LNs when copy number variation is not considered (Xie et al. 2018). The results of these three studies were similar to ours. We utilized resected fresh frozen tissues with sufficient volumes and found that both variant types and allele frequencies were similar between primary sites and metastatic LNs for 36 genes. Although previous studies have not indicated the association between genetic heterogeneity or homogeneity on prognosis, we demonstrated the clinical usefulness of genetic similarity for predicting RFS and PRS. Our results suggested clinical usefulness on the genetic variant-based stratification of nodal-positive cases.
Previous studies have proposed classification methodologies based on characteristics of resected metastatic LNs, for example, the number, ratio, or zone of metastatic LNs (Wei et al. 2011; Jonnalagadda et al. 2011; Saji et al. 2011; Wisnivesky et al. 2011; Rusch et al. 2007, 2009). They assessed status of the whole metastatic LNs. Genetic analyses of all resected LNs are not clinically practical. However, our results suggested that analysis of whole LNs is not necessary, as long as the genetic status of primary site is evaluated. In current study, EGFR mutation and ALK rearrangement are related to worse RFS. Regarding to proposed methodologies, the ratio of metastatic LNs was significantly related to poorer RFS but not PRS. The number or zone of metastatic LNs was not a significant predictor of worse RFS or PRS. Moreover, these previously proposed methodologies have various limitations. For example, precise counts of LNs are often difficult to obtain due to adhesion among LNs. Intraoperative location of LNs can differ even among expert thoracic surgeons (Watanabe et al. 2002). Systematic LN resection can be omitted if LN metastasis is not proven intraoperatively. The advantages of nodal classification by genetic status are that this approach is qualitative, reproducible, and does not necessarily require precise counts or locations of metastatic LNs. Moreover, the status is directly relevant to the choice of treatment after recurrence. Among 31 recurrent cases harbouring EGFR mutation or ALK rearrangement, 27 cases (87.1%) received TKI treatment and PRS was prolonged.
EGFR mutation and ALK rearrangement are driver genetic variants and target of molecular therapy. We previously suggested EGFR mutations are associated to increased risk of recurrence as driver mutations in invasive lung adenocarcinoma (Ito et al. 2018). ALK rearrangement is suggested to be associated with shorter disease-free survival in resected lung adenocarcinoma (Gao et al. 2017; Chaft et al. 2018). In the current study, genetic status at the recurrent site was not confirmed. The indication of TKIs were determined based on the genetic status of the primary site and PRS was prolonged by TKI therapies. This suggests that targetable driver variants are conserved not only in metastatic LN but at the site of recurrence. OS was not prolonged by TKI therapy, in part owing to the small number of cases and previous generation TKIs were used. Considering that newer TKIs have been developed with better therapeutic impact compared to the former-generation TKIs (Soria et al. 2018; Camidge et al. 2018), it is possible that a significant difference in OS will be reached in another cohort that includes more cases receiving newer TKIs.
This study had some limitations. This was a single-institution retrospective study with a small number of cases. EGFR mutations could not be confirmed in metastatic LN in one case, despite the positive status at the primary site. Probably due to the sampling error; the case included metastasis only on one LN. We have not evaluated whether a targetable variant confirmed at one metastatic site could be confirmed at all other metastatic sites. Further exploration is warranted to evaluate whether nodal classification based on genetic status is possible only from the metastatic site status. Additionally, the validation of mutation-based classification and the impact on OS should be evaluated by multi-centre, prospective studies including a large number of cases.
Conclusions
Similar genetic statuses were confirmed in primary sites and metastatic LNs. Cases harbouring targetable genetic variants had a higher risk of recurrence, but post-recurrence survival was prolonged by TKI therapy. Stratification of nodal-positive cases based on targetable genetic variants can be clinically useful for the prediction of recurrence and choice of treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was partially conducted at the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University (Hiroshima, Japan). The authors would like to thank Editage (http://www.editage.jp) for English language editing.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI Grant number 15K19938) and ESTS-ACECP travelling fellowship 2013.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS et al (2018) Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 379:2027–2039. 10.1056/NEJMoa1810171 [DOI] [PubMed] [Google Scholar]
- Chaft JE, Dagogo-Jack I, Santini FC, Eng J, Yeap BY, Izar B et al (2018) Clinical outcomes of patients with resected, early-stage ALK-positive lung cancer. Lung Cancer 122:67–71. 10.1016/j.lungcan.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortot AB, Italiano A, Burel-Vandenbos F, Martel-Planche G, Hainaut P (2010) KRAS mutation status in primary nonsmall cell lung cancer and matched metastases. Cancer 116:2682–2687. 10.1002/cncr.25014 [DOI] [PubMed] [Google Scholar]
- Gao Q, Li P, Jiang X, Zhan Z, Yan Q, Zhang B et al (2017) Worse disease-free, tumor-specific, and overall survival in surgically-resected lung adenocarcinoma patients with ALK rearrangement. Oncotarget 8:86066–86081. 10.18632/oncotarget.20973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yamashita Y, Tsutani Y, Misumi K, Harada H, Miyata Y et al (2013) Classifications of n2 non-small-cell lung cancer based on the number and rate of metastatic mediastinal lymph nodes. Clin Lung Cancer 14:651–657. 10.1016/j.cllc.2013.04.012 [DOI] [PubMed] [Google Scholar]
- Ito M, Miyata Y, Hirano S, Kimura S, Irisuna F, Ikeda K et al (2017) Therapeutic strategies and genetic profile comparisons in small cell carcinoma and large cell neuroendocrine carcinoma of the lung using next-generation sequencing. Oncotarget 8:108936–108945. 10.18632/oncotarget.22426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Miyata Y, Kushitani K, Yoshiya T, Kai Y, Tsutani Y et al (2018) Increased risk of recurrence in resected EGFR-positive pN0M0 invasive lung adenocarcinoma. Thorac Cancer 9:1594–1602. 10.1111/1759-7714.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JH, Kang CH, Kim HS, Seong YW, Park IK, Kim YT (2015) Prognostic and predictive role of epidermal growth factor receptor mutation in recurrent pulmonary adenocarcinoma after curative resection. Eur J Cardiothorac Surg 47:556–562. 10.1093/ejcts/ezu177 [DOI] [PubMed] [Google Scholar]
- Jonnalagadda S, Smith C, Mhango G, Wisnivesky JP (2011) The number of lymph node metastases as a prognostic factor in patients with N1 non-small cell lung cancer. Chest 140:433–440. 10.1378/chest.10-2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB et al (2018) Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol 36:911–919. 10.1200/JCO.2017.76.7293 [DOI] [PubMed] [Google Scholar]
- Kalikaki A, Koutsopoulos A, Trypaki M, Souglakos J, Stathopoulos E, Georgoulias V et al (2008) Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 99:923–929. 10.1038/sj.bjc.6604629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GD, Kim DK, Moon DH, Joo S, Hwang SK, Choi SH et al (2016) A comparison of the proposed classifications for the revision of N descriptors for non-small-cell lung cancer. Eur J Cardiothorac Surg 49:580–588. 10.1093/ejcts/ezv134 [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Takahashi K, Iwakawa R, Matsuno Y, Nakanishi Y, Kohno T et al (2006) Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer 119:1491–1494. 10.1002/ijc.21940 [DOI] [PubMed] [Google Scholar]
- Nagai Y, Miyazawa H, Huqun Tanaka T, Udagawa K, Kato M et al (2005) Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res 65:7276–7282. 10.1158/0008-5472.CAN-05-0331 [DOI] [PubMed] [Google Scholar]
- Park S, Holmes-Tisch AJ, Cho EY, Shim YM, Kim J, Kim HS et al (2009) Discordance of molecular biomarkers associated with epidermal growth factor receptor pathway between primary tumors and lymph node metastasis in non-small cell lung cancer. J Thorac Oncol 4:809–815. 10.1097/JTO.0b013e3181a94af4 [DOI] [PubMed] [Google Scholar]
- Rami-Porta R, Call S, Dooms C, Obiols C, Sánchez M, Travis WD et al (2018) Lung cancer staging: a concise update. Eur Respir J 17:1800190. 10.1183/13993003.00190-2018 [DOI] [PubMed] [Google Scholar]
- Rusch VW, Crowley J, Giroux DJ, Goldstraw P, Im JG, Tsuboi M et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2:603–612. 10.1097/JTO.0b013e31807ec803 [DOI] [PubMed] [Google Scholar]
- Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P et al (2009) The IASLC Lung Cancer Staging Project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 4:568–577. 10.1097/JTO.0b013e3181a0d82e [DOI] [PubMed] [Google Scholar]
- Saji H, Tsuboi M, Yoshida K, Kato Y, Nomura M, Matsubayashi J et al (2011) Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 6:1865–1871. 10.1097/JTO.0b013e31822a35c3 [DOI] [PubMed] [Google Scholar]
- Schmid K, Oehl N, Wrba F, Pirker R, Pirker C, Filipits M (2009) EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res 15:4554–4560. 10.1158/1078-0432.CCR-09-0089 [DOI] [PubMed] [Google Scholar]
- Shimada Y, Saji H, Yoshida K, Kakihana M, Honda H, Nomura M et al (2013) Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 143:1626–1634. 10.1378/chest.12-1717 [DOI] [PubMed] [Google Scholar]
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113–125. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- Takenaka T, Takenoyama M, Yamaguchi M, Toyozawa R, Inamasu E, Kojo M et al (2015) Impact of the epidermal growth factor receptor mutation status on the post-recurrence survival of patients with surgically resected non-small-cell lung cancer. Eur J Cardiothorac Surg 47:550–555. 10.1093/ejcts/ezu227 [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6:244–285. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Nicholson A et al (2015) The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10(9):1243–1260 [DOI] [PubMed] [Google Scholar]
- Vignot S, Frampton GM, Soria JC, Yelensky R, Commo F, Brambilla C et al (2013) Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol 31:2167–2172. 10.1200/JCO.2012.47.7737 [DOI] [PubMed] [Google Scholar]
- Watanabe S, Ladas G, Goldstraw P (2002) Inter-observer variability in systematic nodal dissection: comparison of European and Japanese nodal designation. Ann Thorac Surg 73:245–248 (discussion 248–249) [DOI] [PubMed] [Google Scholar]
- Wei S, Asamura H, Kawachi R, Sakurai H, Watanabe S (2011) Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 6:310–318. 10.1097/JTO.0b013e3181ff9b45 [DOI] [PubMed] [Google Scholar]
- Wisnivesky JP, Arciniega J, Mhango G, Mandeli J, Halm EA (2011) Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax 66:287–293. 10.1136/thx.2010.148601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Zhang Y, Mao X, Zheng X, Han-Zhang H, Ye J et al (2018) Comparison of genetic profiles among primary lung tumor, metastatic lymph nodes and circulating tumor DNA in treatment-naïve advanced non-squamous non-small cell lung cancer patients. Lung Cancer 121:54–60. 10.1016/j.lungcan.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Matsuo K, Mitsudomi T (2011) Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 29:2972–2977. 10.1200/JCO.2010.33.3906 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.