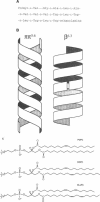
Abstract
In organic solvents gramicidin A (gA) occurs as a mixture of slowly interconverting double-stranded dimers. Membrane-spanning gA channels, in contrast, are almost exclusively single-stranded beta(6,3)-helical dimers. Based on spectroscopic evidence, it has previously been concluded that the conformational preference of gA in phospholipid bilayers varies as a function of the degree of unsaturation of the acyl chains. Double-stranded pi pi(5,6)-helical dimers predominate (over single-stranded beta(6,3)-helical dimers) in lipid bilayer membranes with polyunsaturated acyl chains. We therefore examined the characteristics of channels formed by gA in 1-palmitoyl-2-oleoylphosphatidylcholine/n-decane, 1,2-dioleoylphosphatidylcholine/n-decane, and 1,2-dilinoleoylphosphatidylcholine/n-decane bilayers. We did not observe long-lived channels that could be conducting double-stranded pi pi(5,6)-helical dimers in any of these different membrane environments. We conclude that the single-stranded beta(6,3)-helical dimer is the only conducting species in these bilayers. Somewhat surprisingly, the average channel duration and channel-forming potency of gA are increased in dilinoleoylphosphatidylcholine/n-decane bilayers compared to 1-palmitoyl-2-oleoylphosphatidylcholine/n-decane and dioleoylphosphatidylcholine/n-decane bilayers. To test for specific interactions between the aromatic side chains of gA and the acyl chains of the bilayer, we examined the properties of channels formed by gramicidin analogues in which the four tryptophan residues were replaced with naphthylalanine (gN), tyrosine (gT), and phenylalanine (gM). The results show that all of these analogue channels experience the same relative stabilization when going from dioleoylphosphatidylcholine to dilinoleoylphosphatidylcholine bilayers.
Full text
PDF
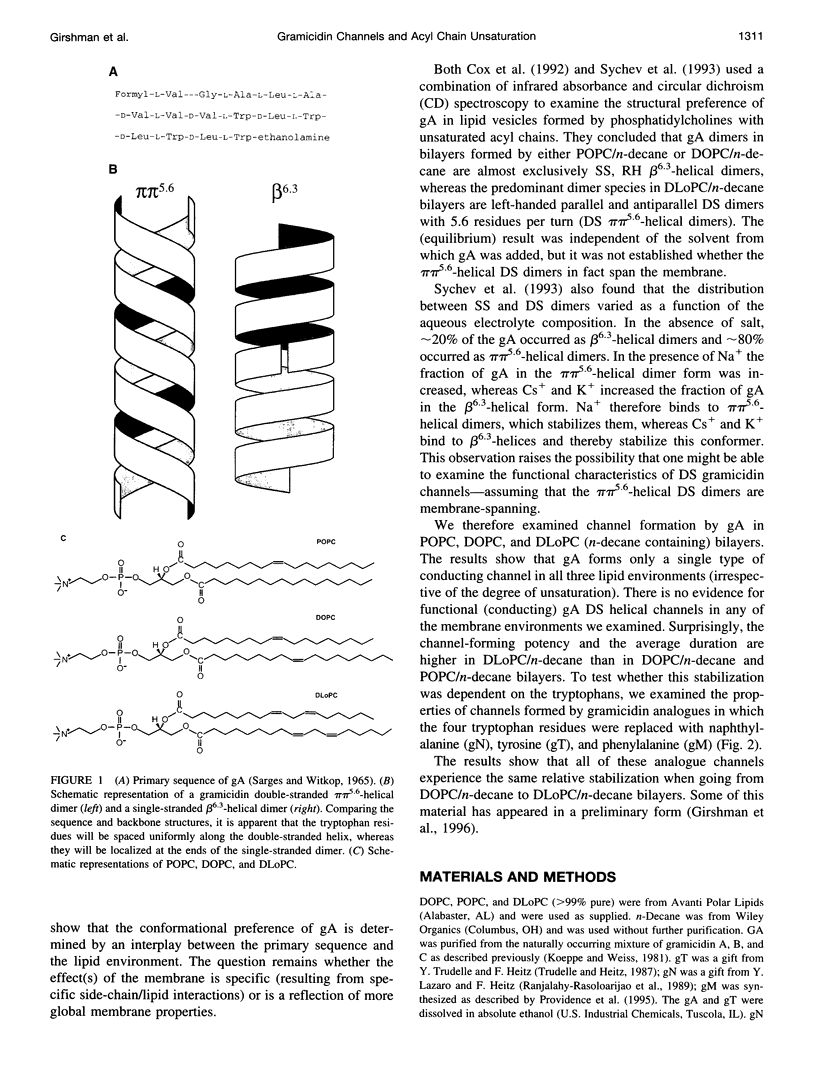
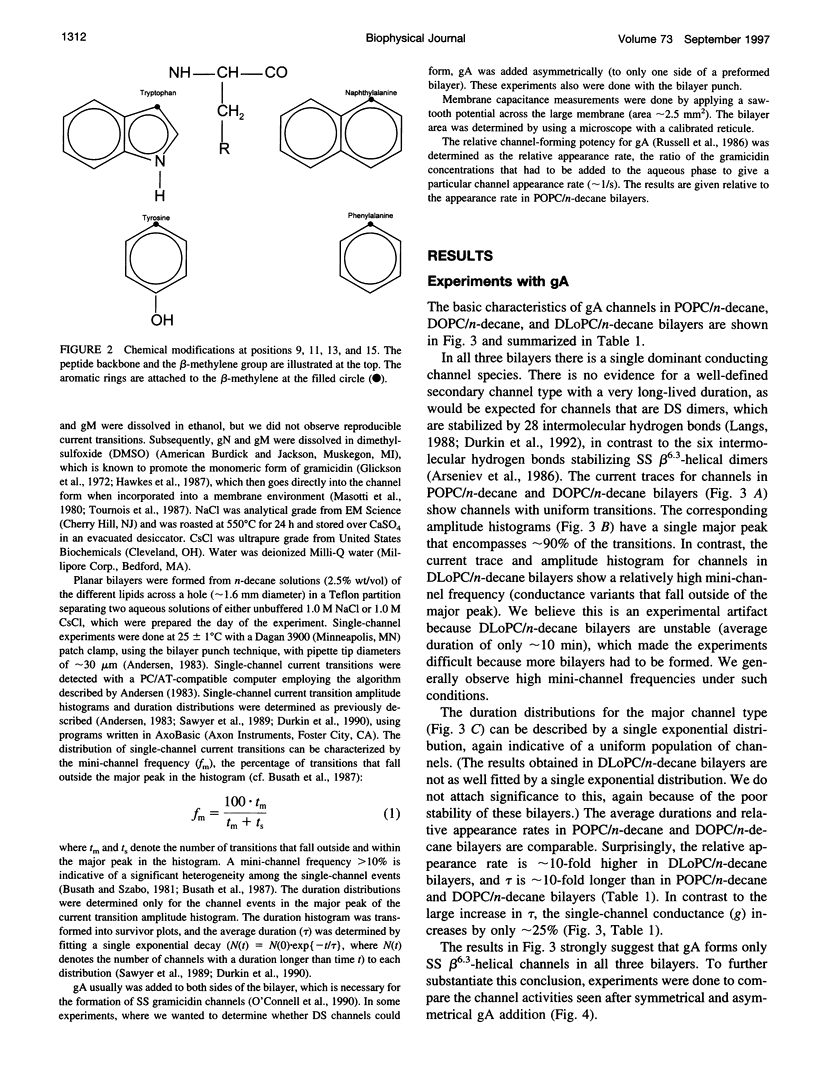
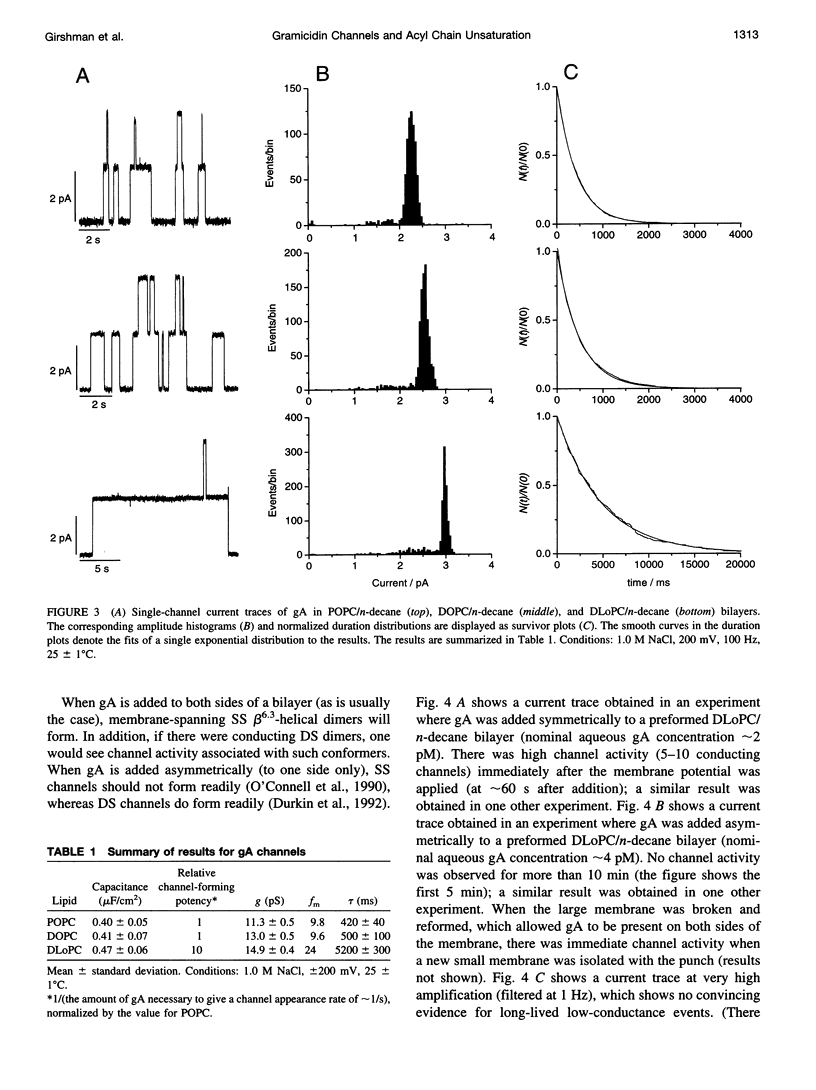





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Manan N., Hinton J. F. Conformation states of gramicidin A along the pathway to the formation of channels in model membranes determined by 2D NMR and circular dichroism spectroscopy. Biochemistry. 1994 Jun 7;33(22):6773–6783. doi: 10.1021/bi00188a005. [DOI] [PubMed] [Google Scholar]
- Andersen O. S. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys J. 1983 Feb;41(2):119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Koeppe R. E., 2nd Molecular determinants of channel function. Physiol Rev. 1992 Oct;72(4 Suppl):S89–158. doi: 10.1152/physrev.1992.72.suppl_4.S89. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Channel formation kinetics of gramicidin A in lipid bilayer membranes. J Membr Biol. 1973;11(2):177–194. doi: 10.1007/BF01869820. [DOI] [PubMed] [Google Scholar]
- Bañ M. C., Braco L., Abad C. New high-performance liquid chromatography-based methodology for monitoring the conformational transitions of self-associating hydrophobic peptides, incorporated into liposomes. J Chromatogr. 1988 Dec 23;458:105–116. doi: 10.1016/s0021-9673(00)90557-0. [DOI] [PubMed] [Google Scholar]
- Becker M. D., Greathouse D. V., Koeppe R. E., 2nd, Andersen O. S. Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration. Biochemistry. 1991 Sep 10;30(36):8830–8839. doi: 10.1021/bi00100a015. [DOI] [PubMed] [Google Scholar]
- Busath D. D., Andersen O. S., Koeppe R. E., 2nd On the conductance heterogeneity in membrane channels formed by gramicidin A. A cooperative study. Biophys J. 1987 Jan;51(1):79–88. doi: 10.1016/S0006-3495(87)83313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busath D., Szabo G. Gramicidin forms multi-state rectifying channels. Nature. 1981 Nov 26;294(5839):371–373. doi: 10.1038/294371a0. [DOI] [PubMed] [Google Scholar]
- Cifu A. S., Koeppe R. E., 2nd, Andersen O. S. On the supramolecular organization of gramicidin channels. The elementary conducting unit is a dimer. Biophys J. 1992 Jan;61(1):189–203. doi: 10.1016/S0006-3495(92)81826-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. J., Ho C., Lombardi J. V., Stubbs C. D. Gramicidin conformational studies with mixed-chain unsaturated phospholipid bilayer systems. Biochemistry. 1992 Feb 4;31(4):1112–1117. doi: 10.1021/bi00119a020. [DOI] [PubMed] [Google Scholar]
- Dilger J. P., McLaughlin S. G., McIntosh T. J., Simon S. A. The dielectric constant of phospholipid bilayers and the permeability of membranes to ions. Science. 1979 Dec 7;206(4423):1196–1198. doi: 10.1126/science.228394. [DOI] [PubMed] [Google Scholar]
- Doyle D. A., Wallace B. A. Crystal structure of the gramicidin/potassium thiocyanate complex. J Mol Biol. 1997 Mar 14;266(5):963–977. doi: 10.1006/jmbi.1996.0837. [DOI] [PubMed] [Google Scholar]
- Durkin J. T., Koeppe R. E., 2nd, Andersen O. S. Energetics of gramicidin hybrid channel formation as a test for structural equivalence. Side-chain substitutions in the native sequence. J Mol Biol. 1990 Jan 5;211(1):221–234. doi: 10.1016/0022-2836(90)90022-E. [DOI] [PubMed] [Google Scholar]
- Durkin J. T., Providence L. L., Koeppe R. E., 2nd, Andersen O. S. Formation of non-beta 6.3-helical gramicidin channels between sequence-substituted gramicidin analogues. Biophys J. 1992 Apr;62(1):145–159. doi: 10.1016/S0006-3495(92)81801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Needham D., Dilger J. P., Haydon D. A. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim Biophys Acta. 1983 Oct 26;735(1):95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- Fonseca V., Daumas P., Ranjalahy-Rasoloarijao L., Heitz F., Lazaro R., Trudelle Y., Andersen O. S. Gramicidin channels that have no tryptophan residues. Biochemistry. 1992 Jun 16;31(23):5340–5350. doi: 10.1021/bi00138a014. [DOI] [PubMed] [Google Scholar]
- Glickson J. D., Mayers D. F., Settine J. M., Urry D. W. Spectroscopic studies on the conformation of gramicidin A'. Proton magnetic resonance assignments, coupling constants, and H-D exchange. Biochemistry. 1972 Feb 15;11(4):477–486. doi: 10.1021/bi00754a001. [DOI] [PubMed] [Google Scholar]
- Greathouse D. V., Hinton J. F., Kim K. S., Koeppe R. E., 2nd Gramicidin A/short-chain phospholipid dispersions: chain length dependence of gramicidin conformation and lipid organization. Biochemistry. 1994 Apr 12;33(14):4291–4299. doi: 10.1021/bi00180a025. [DOI] [PubMed] [Google Scholar]
- Hawkes G. E., Lian L. Y., Randall E. W., Sales K. D., Curzon E. H. The conformation of gramicidin A in dimethylsulphoxide solution. A full analysis of the one- and two-dimensional 1H, 13C, and 15N nuclear-magnetic-resonance spectra. Eur J Biochem. 1987 Jul 15;166(2):437–445. doi: 10.1111/j.1432-1033.1987.tb13535.x. [DOI] [PubMed] [Google Scholar]
- Heitz F., Spach G., Trudelle Y. Single channels of 9, 11, 13, 15-destryptophyl-phenylalanyl-gramicidin A. Biophys J. 1982 Oct;40(1):87–89. doi: 10.1016/S0006-3495(82)84462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich P., Jakobsson E. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophys J. 1990 May;57(5):1075–1084. doi: 10.1016/S0006-3495(90)82625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Huang H. W. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys J. 1986 Dec;50(6):1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C. Energy barriers for passage of ions through channels. Exact solution of two electrostatic problems. Biophys Chem. 1981 Jun;13(3):203–212. doi: 10.1016/0301-4622(81)80002-6. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Burger K. N., de Kruijff B. Phase separation and hexagonal HII phase formation by gramicidins A, B and C in dioleoylphosphatidylcholine model membranes. A study on the role of the tryptophan residues. Biochim Biophys Acta. 1987 Feb 26;897(2):269–284. doi: 10.1016/0005-2736(87)90423-8. [DOI] [PubMed] [Google Scholar]
- Killian J. A. Gramicidin and gramicidin-lipid interactions. Biochim Biophys Acta. 1992 Dec 11;1113(3-4):391–425. doi: 10.1016/0304-4157(92)90008-x. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Prasad K. U., Urry D. W., de Kruijff B. A mismatch between the length of gramicidin and the lipid acyl chains is a prerequisite for HII phase formation in phosphatidylcholine model membranes. Biochim Biophys Acta. 1989 Jan 30;978(2):341–345. doi: 10.1016/0005-2736(89)90135-1. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Timmermans J. W., Keur S., de Kruijff B. The tryptophans of gramicidin are essential for the lipid structure modulating effect of the peptide. Biochim Biophys Acta. 1985 Oct 24;820(1):154–156. doi: 10.1016/0005-2736(85)90227-5. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Anderson O. S. Engineering the gramicidin channel. Annu Rev Biophys Biomol Struct. 1996;25:231–258. doi: 10.1146/annurev.bb.25.060196.001311. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Providence L. L., Greathouse D. V., Heitz F., Trudelle Y., Purdie N., Andersen O. S. On the helix sense of gramicidin A single channels. Proteins. 1992 Jan;12(1):49–62. doi: 10.1002/prot.340120107. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Bamberg E. Influence of membrane thickness and ion concentration on the properties of the gramicidin a channel. Autocorrelation, spectral power density, relaxation and single-channel studies. Biochim Biophys Acta. 1977 Jan 4;464(1):127–141. doi: 10.1016/0005-2736(77)90376-5. [DOI] [PubMed] [Google Scholar]
- Langs D. A. Three-dimensional structure at 0.86 A of the uncomplexed form of the transmembrane ion channel peptide gramicidin A. Science. 1988 Jul 8;241(4862):188–191. doi: 10.1126/science.2455345. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Lundbaek J. A., Andersen O. S. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol. 1994 Oct;104(4):645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masotti L., Spisni A., Urry D. W. Conformational studies on the gramicidin A transmembrane channel in lipid micelles and liposomes. Cell Biophys. 1980 Sep;2(3):241–251. doi: 10.1007/BF02790452. [DOI] [PubMed] [Google Scholar]
- Nicholson L. K., Cross T. A. Gramicidin cation channel: an experimental determination of the right-handed helix sense and verification of beta-type hydrogen bonding. Biochemistry. 1989 Nov 28;28(24):9379–9385. doi: 10.1021/bi00450a019. [DOI] [PubMed] [Google Scholar]
- O'Connell A. M., Koeppe R. E., 2nd, Andersen O. S. Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science. 1990 Nov 30;250(4985):1256–1259. doi: 10.1126/science.1700867. [DOI] [PubMed] [Google Scholar]
- Pascal S. M., Cross T. A. Structure of an isolated gramicidin A double helical species by high-resolution nuclear magnetic resonance. J Mol Biol. 1992 Aug 20;226(4):1101–1109. doi: 10.1016/0022-2836(92)91055-t. [DOI] [PubMed] [Google Scholar]
- Providence L. L., Andersen O. S., Greathouse D. V., Koeppe R. E., 2nd, Bittman R. Gramicidin channel function does not depend on phospholipid chirality. Biochemistry. 1995 Dec 19;34(50):16404–16411. doi: 10.1021/bi00050a022. [DOI] [PubMed] [Google Scholar]
- Ranjalahy-Rasoloarijao L., Lazaro R., Daumas P., Heitz F. Synthesis and ionic channels of a linear gramicidin containing naphthylalanine instead of tryptophan. Int J Pept Protein Res. 1989 Apr;33(4):273–280. doi: 10.1111/j.1399-3011.1989.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Roux B., Brüschweiler R., Ernst R. R. The structure of gramicidin A in dimethylsulfoxide/acetone. Eur J Biochem. 1990 Nov 26;194(1):57–60. doi: 10.1111/j.1432-1033.1990.tb19426.x. [DOI] [PubMed] [Google Scholar]
- Russell E. W., Weiss L. B., Navetta F. I., Koeppe R. E., 2nd, Andersen O. S. Single-channel studies on linear gramicidins with altered amino acid side chains. Effects of altering the polarity of the side chain at position 1 in gramicidin A. Biophys J. 1986 Mar;49(3):673–686. doi: 10.1016/S0006-3495(86)83694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARGES R., WITKOP B. GRAMICIDIN A. V. THE STRUCTURE OF VALINE- AND ISOLEUCINE-GRAMICIDIN A. J Am Chem Soc. 1965 May 5;87:2011–2020. doi: 10.1021/ja01087a027. [DOI] [PubMed] [Google Scholar]
- Sackmann E., Kotulla R., Heiszler F. J. On the role of lipid-bilayer elasticity for the lipid-protein interaction and the indirect protein-protein coupling. Can J Biochem Cell Biol. 1984 Aug;62(8):778–788. doi: 10.1139/o84-099. [DOI] [PubMed] [Google Scholar]
- Sawyer D. B., Koeppe R. E., 2nd, Andersen O. S. Induction of conductance heterogeneity in gramicidin channels. Biochemistry. 1989 Aug 8;28(16):6571–6583. doi: 10.1021/bi00442a007. [DOI] [PubMed] [Google Scholar]
- Sychev S. V., Barsukov L. I., Ivanov V. T. The double pi pi 5.6 helix of gramicidin A predominates in unsaturated lipid membranes. Eur Biophys J. 1993;22(4):279–288. doi: 10.1007/BF00180262. [DOI] [PubMed] [Google Scholar]
- Tournois H., Killian J. A., Urry D. W., Bokking O. R., de Gier J., de Kruijff B. Solvent determined conformation of gramicidin affects the ability of the peptide to induce hexagonal HII phase formation in dioleoylphosphatidylcholine model membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):222–226. doi: 10.1016/0005-2736(87)90026-5. [DOI] [PubMed] [Google Scholar]
- Trudelle Y., Heitz F. Synthesis and characterization of Tyr(Bzl)9,11,13,15 and Tyr9,11,13,15 gramicidin A. Int J Pept Protein Res. 1987 Aug;30(2):163–169. doi: 10.1111/j.1399-3011.1987.tb03325.x. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Fossel E. T., Blout E. R. The conformation of gramicidin A. Biochemistry. 1974 Dec 17;13(26):5249–5256. doi: 10.1021/bi00723a001. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Ravikumar K. The gramicidin pore: crystal structure of a cesium complex. Science. 1988 Jul 8;241(4862):182–187. doi: 10.1126/science.2455344. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Pascal S. M., Cross T. A. A conformational rearrangement in gramicidin A: from a double-stranded left-handed to a single-stranded right-handed helix. Biochemistry. 1992 Sep 22;31(37):8822–8828. doi: 10.1021/bi00152a019. [DOI] [PubMed] [Google Scholar]