Abstract
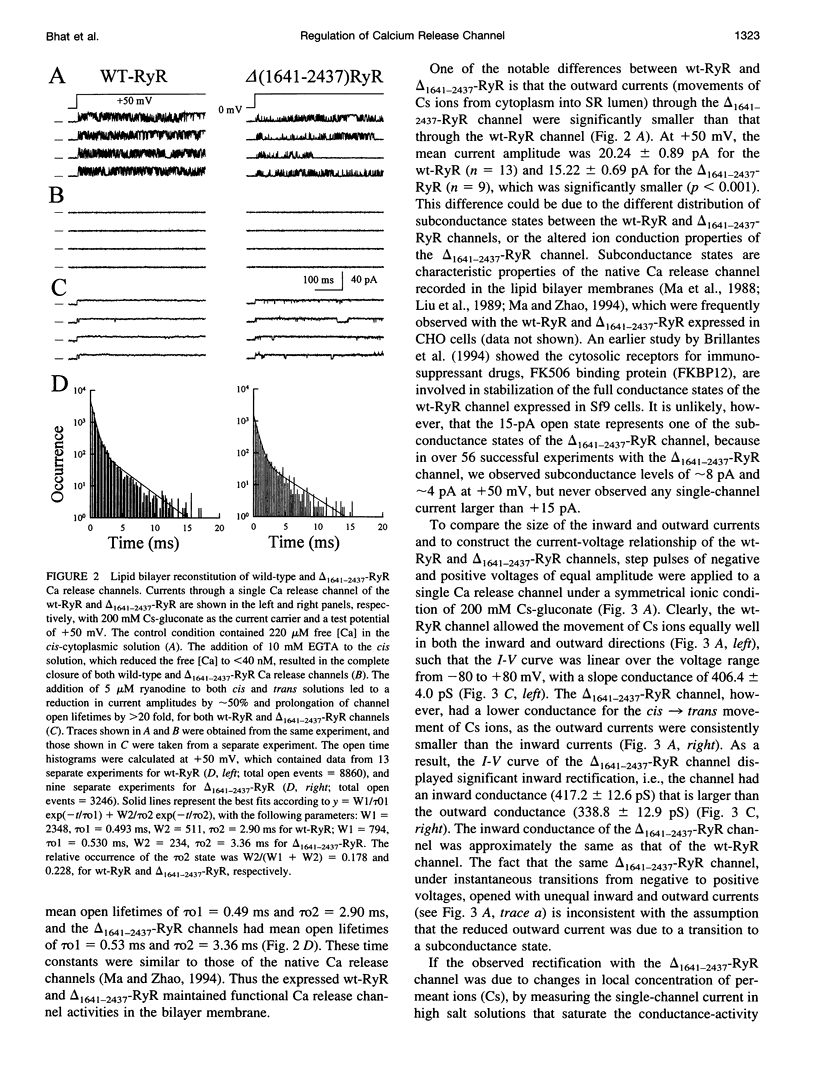
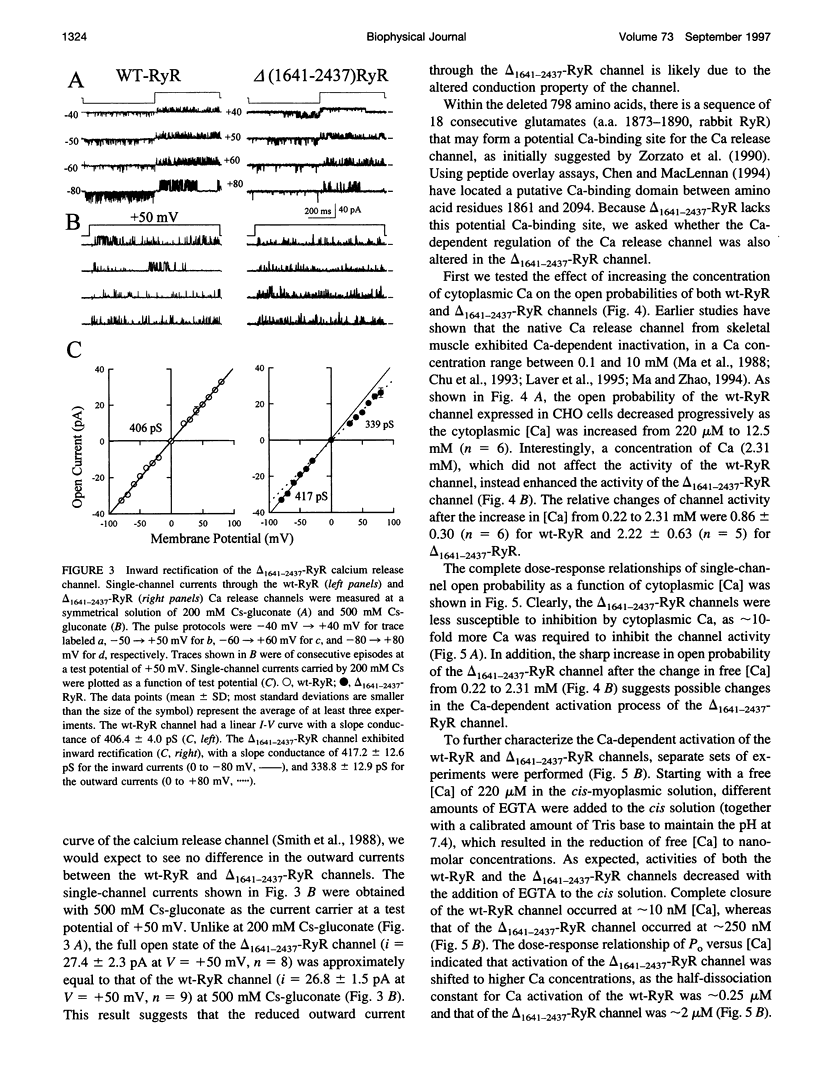
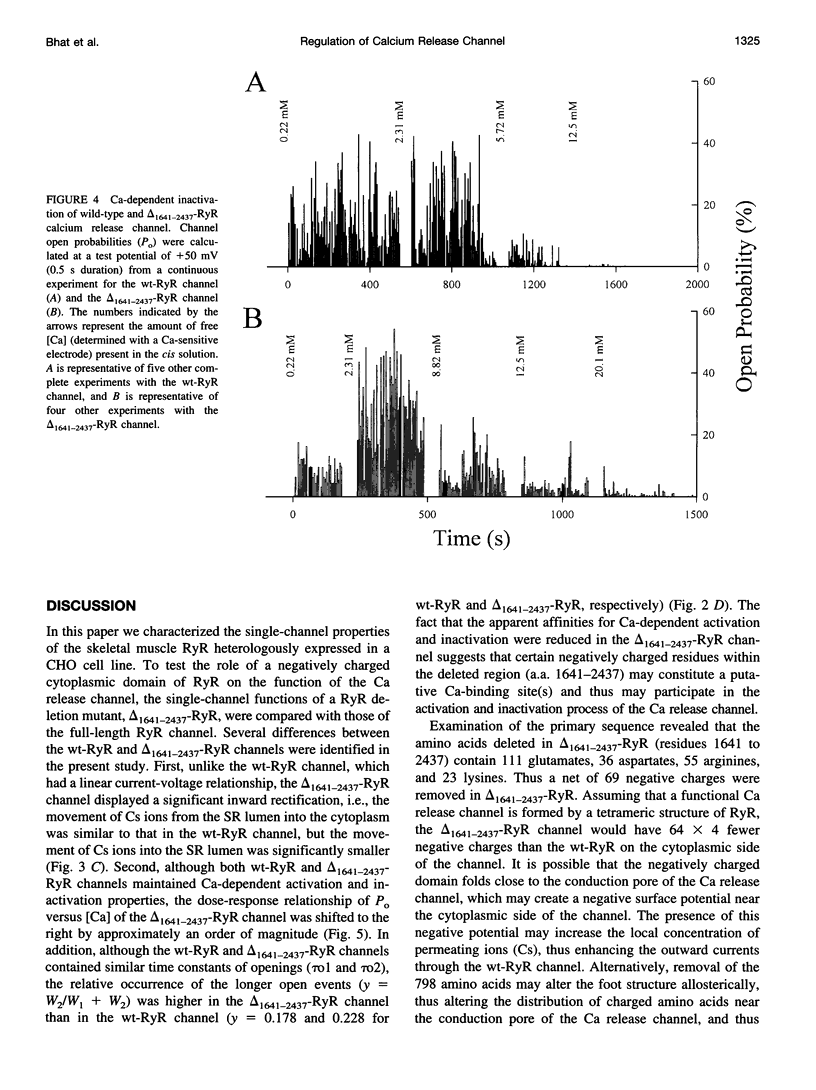
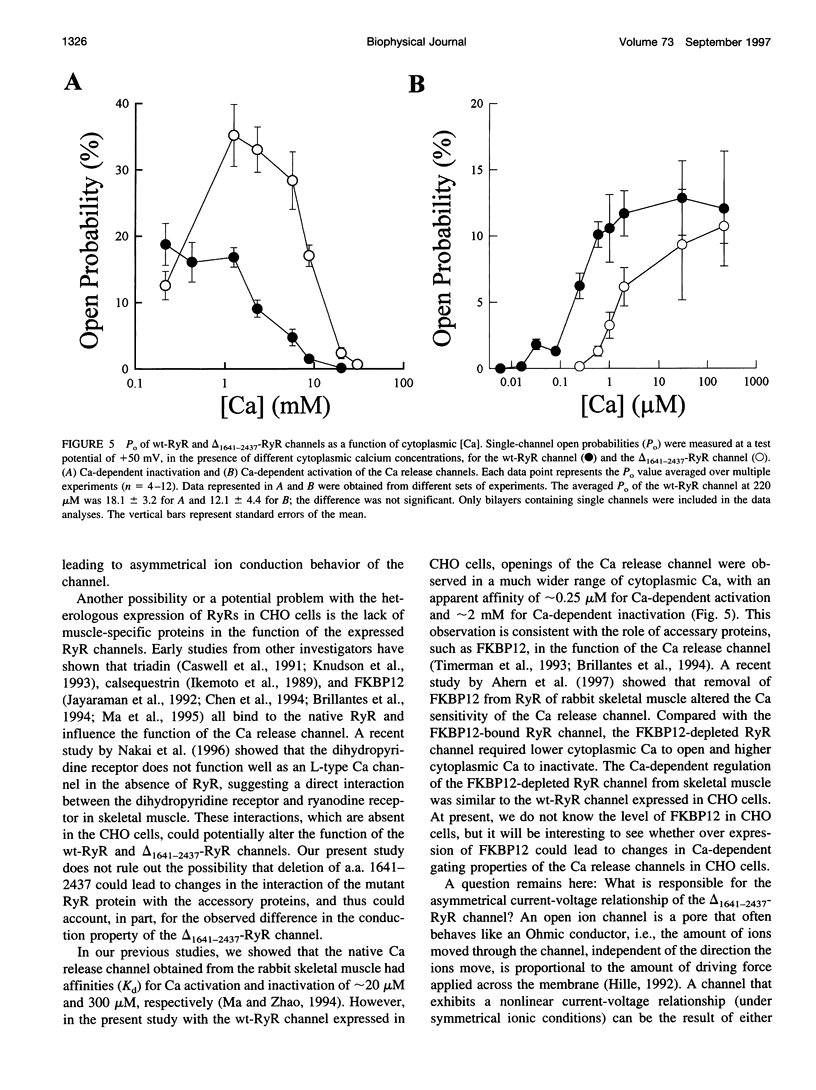
The ryanodine receptor (RyR) of skeletal muscle contains two functional domains: a carboxyl-terminal hydrophobic domain that forms the putative conduction pore of the calcium release channel, and a large cytoplasmic domain that corresponds to the "foot structure." To understand the contribution of the foot structure to the function of the calcium release channel, we studied a RyR deletion mutant, delta(1641-2437)-RyR, in which a region that is rich in glutamate and aspartate residues (a.a. 1641-2437) was removed. The wild-type and delta(1641-2437)-RyR proteins were expressed in a Chinese hamster ovary (CHO) cell line, and functions of single calcium release channels were measured in the lipid bilayer membrane. The wild-type RyR forms functional calcium release channels with a linear current-voltage relationship similar to that of the native channel identified in the sarcoplasmic reticulum membrane of skeletal muscle, whereas the channels formed by delta(1641-2437)-RyR exhibit significant inward rectification, i.e., currents moving from cytoplasm into SR lumen were approximately 20% less than that in the opposite direction. As in to the wt-RyR channel, opening of the delta(1641-2437)-RyR channel has a bell-shaped dependence on the cytoplasmic calcium, but the calcium-dependent activation and inactivation processes of the delta(1641-2437)-RyR channel are shifted to higher calcium concentrations. Our data show that deletion of a.a. 1641-2437 from the foot region of the skeletal muscle RyR results in changes in both ion conduction and calcium-dependent regulation of the calcium release channel.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern G. P., Junankar P. R., Dulhunty A. F. Subconductance states in single-channel activity of skeletal muscle ryanodine receptors after removal of FKBP12. Biophys J. 1997 Jan;72(1):146–162. doi: 10.1016/S0006-3495(97)78654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
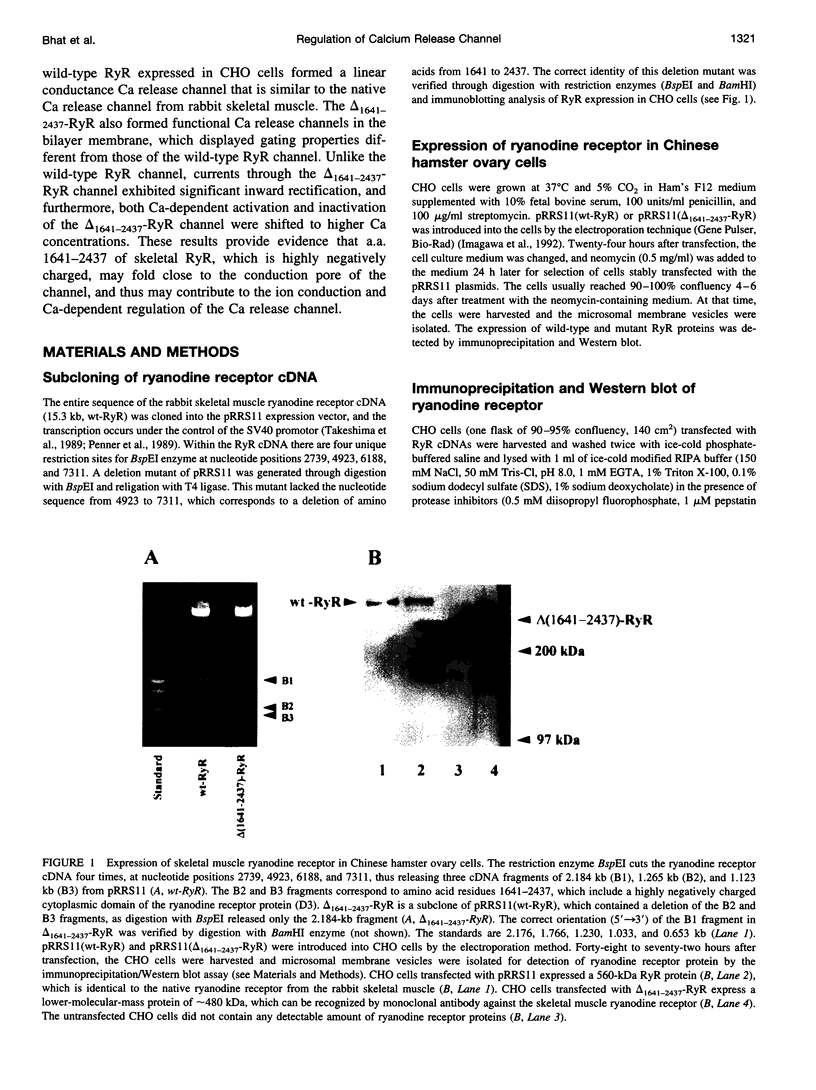
- Bhat M. B., Zhao J., Takeshima H., Ma J. Functional calcium release channel formed by the carboxyl-terminal portion of ryanodine receptor. Biophys J. 1997 Sep;73(3):1329–1336. doi: 10.1016/S0006-3495(97)78166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E., Marks A. R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994 May 20;77(4):513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Brandt N. R., Brunschwig J. P., Purkerson S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95,000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry. 1991 Jul 30;30(30):7507–7513. doi: 10.1021/bi00244a020. [DOI] [PubMed] [Google Scholar]
- Chen S. R., MacLennan D. H. Identification of calmodulin-, Ca(2+)-, and ruthenium red-binding domains in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1994 Sep 9;269(36):22698–22704. [PubMed] [Google Scholar]
- Chen S. R., Vaughan D. M., Airey J. A., Coronado R., MacLennan D. H. Functional expression of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum in COS-1 cells. Biochemistry. 1993 Apr 13;32(14):3743–3753. doi: 10.1021/bi00065a029. [DOI] [PubMed] [Google Scholar]
- Chen S. R., Zhang L., MacLennan D. H. Asymmetrical blockade of the Ca2+ release channel (ryanodine receptor) by 12-kDa FK506 binding protein. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11953–11957. doi: 10.1073/pnas.91.25.11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A., Fill M., Stefani E., Entman M. L. Cytoplasmic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca(2+)-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. J Membr Biol. 1993 Jul;135(1):49–59. doi: 10.1007/BF00234651. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Jorgensen A. O. Structure and development of E-C coupling units in skeletal muscle. Annu Rev Physiol. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Ikemoto N., Ronjat M., Mészáros L. G., Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989 Aug 8;28(16):6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Imagawa T., Nakai J., Takeshima H., Nakasaki Y., Shigekawa M. Expression of Ca(2+)-induced Ca2+ release channel activity from cardiac ryanodine receptor cDNA in Chinese hamster ovary cells. J Biochem. 1992 Oct;112(4):508–513. doi: 10.1093/oxfordjournals.jbchem.a123930. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Brillantes A. M., Timerman A. P., Fleischer S., Erdjument-Bromage H., Tempst P., Marks A. R. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992 May 15;267(14):9474–9477. [PubMed] [Google Scholar]
- Knudson C. M., Stang K. K., Moomaw C. R., Slaughter C. A., Campbell K. P. Primary structure and topological analysis of a skeletal muscle-specific junctional sarcoplasmic reticulum glycoprotein (triadin). J Biol Chem. 1993 Jun 15;268(17):12646–12654. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Laver D. R., Roden L. D., Ahern G. P., Eager K. R., Junankar P. R., Dulhunty A. F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol. 1995 Sep;147(1):7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Lai F. A., Rousseau E., Jones R. V., Meissner G. Multiple conductance states of the purified calcium release channel complex from skeletal sarcoplasmic reticulum. Biophys J. 1989 Mar;55(3):415–424. doi: 10.1016/S0006-3495(89)82835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Bhat M. B., Zhao J. Rectification of skeletal muscle ryanodine receptor mediated by FK506 binding protein. Biophys J. 1995 Dec;69(6):2398–2404. doi: 10.1016/S0006-3495(95)80109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Fill M., Knudson C. M., Campbell K. P., Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988 Oct 7;242(4875):99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- Ma J., Zhao J. Highly cooperative and hysteretic response of the skeletal muscle ryanodine receptor to changes in proton concentrations. Biophys J. 1994 Aug;67(2):626–633. doi: 10.1016/S0006-3495(94)80522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks A. R., Tempst P., Hwang K. S., Taubman M. B., Inui M., Chadwick C., Fleischer S., Nadal-Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Nakai J., Dirksen R. T., Nguyen H. T., Pessah I. N., Beam K. G., Allen P. D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996 Mar 7;380(6569):72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Nakai J., Imagawa T., Hakamat Y., Shigekawa M., Takeshima H., Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990 Oct 1;271(1-2):169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Penner R., Neher E., Takeshima H., Nishimura S., Numa S. Functional expression of the calcium release channel from skeletal muscle ryanodine receptor cDNA. FEBS Lett. 1989 Dec 18;259(1):217–221. doi: 10.1016/0014-5793(89)81532-7. [DOI] [PubMed] [Google Scholar]
- Ríos E., Ma J. J., González A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J Muscle Res Cell Motil. 1991 Apr;12(2):127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V., Volpe P. Ryanodine receptors: how many, where and why? Trends Pharmacol Sci. 1993 Mar;14(3):98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H. Primary structure and expression from cDNAs of the ryanodine receptor. Ann N Y Acad Sci. 1993 Dec 20;707:165–177. doi: 10.1111/j.1749-6632.1993.tb38051.x. [DOI] [PubMed] [Google Scholar]
- Timerman A. P., Ogunbumni E., Freund E., Wiederrecht G., Marks A. R., Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993 Nov 5;268(31):22992–22999. [PubMed] [Google Scholar]
- Tu Q., Velez P., Cortes-Gutierrez M., Fill M. Surface charge potentiates conduction through the cardiac ryanodine receptor channel. J Gen Physiol. 1994 May;103(5):853–867. doi: 10.1085/jgp.103.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J., Saito A., Inui M., Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989 Mar 9;338(6211):167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]