Abstract
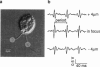
Both phototactic and photophobic responses of Chlamydomonas are mediated by a visual system comprising a rhodopsin photoreceptor. Suction pipette recordings have revealed that flash stimulation causes calcium currents into the eyespot and the flagella. These photocurrents have been suggested to be the trigger for all behavioral light responses of the cell. But this has never been shown experimentally. Here we describe a detection technique that combines electrical and optical measurements from individual algae held in a suction pipette. Thus it is possible to record photocurrents and flagellar beating simultaneously and establish a direct link between the two. We demonstrate that in Chlamydomonas only the photoreceptor current in conjuction with a fast flagellar current constitutes the trigger for photophobic responses. Within the time of the action-potential-like flagellar current, the flagella switch from forward to backward swimming, which constitutes the beginning of the photoshock reaction. The switch is accompanied by a complex frequency change and beating pattern modulation. The results are interpreted in terms of a general model for phototransduction in green algae (Chlorophyceae).
Full text
PDF
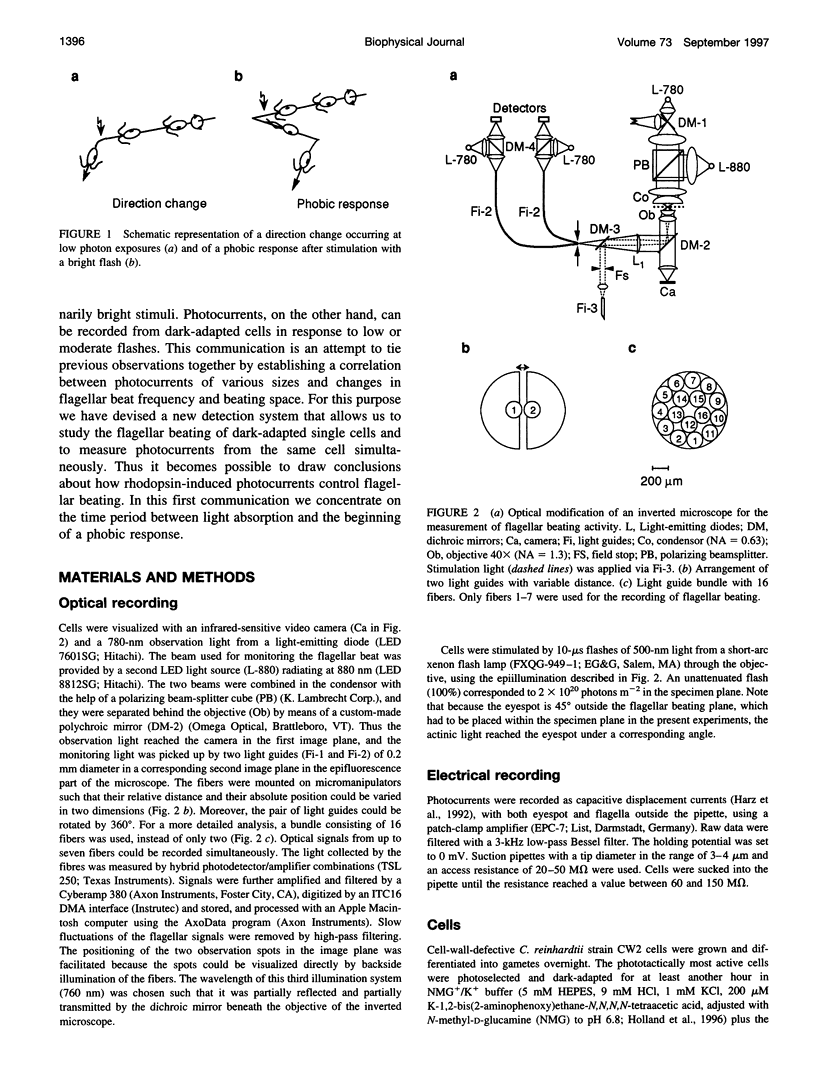
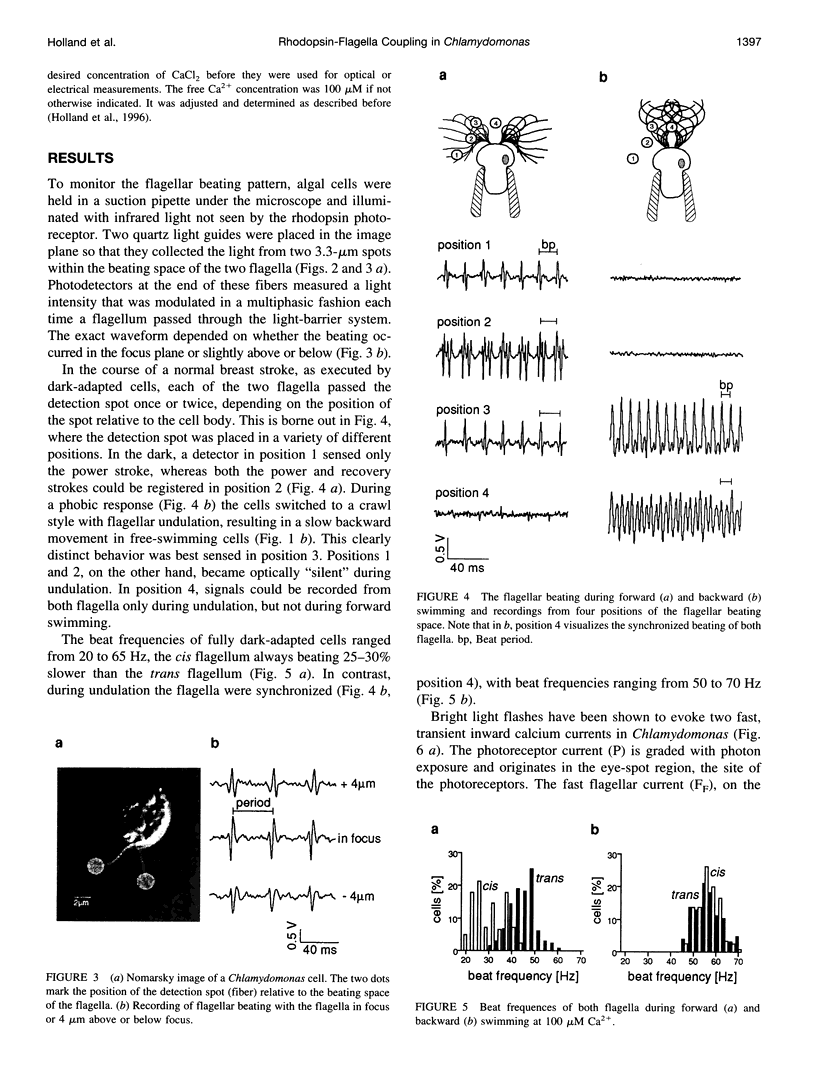

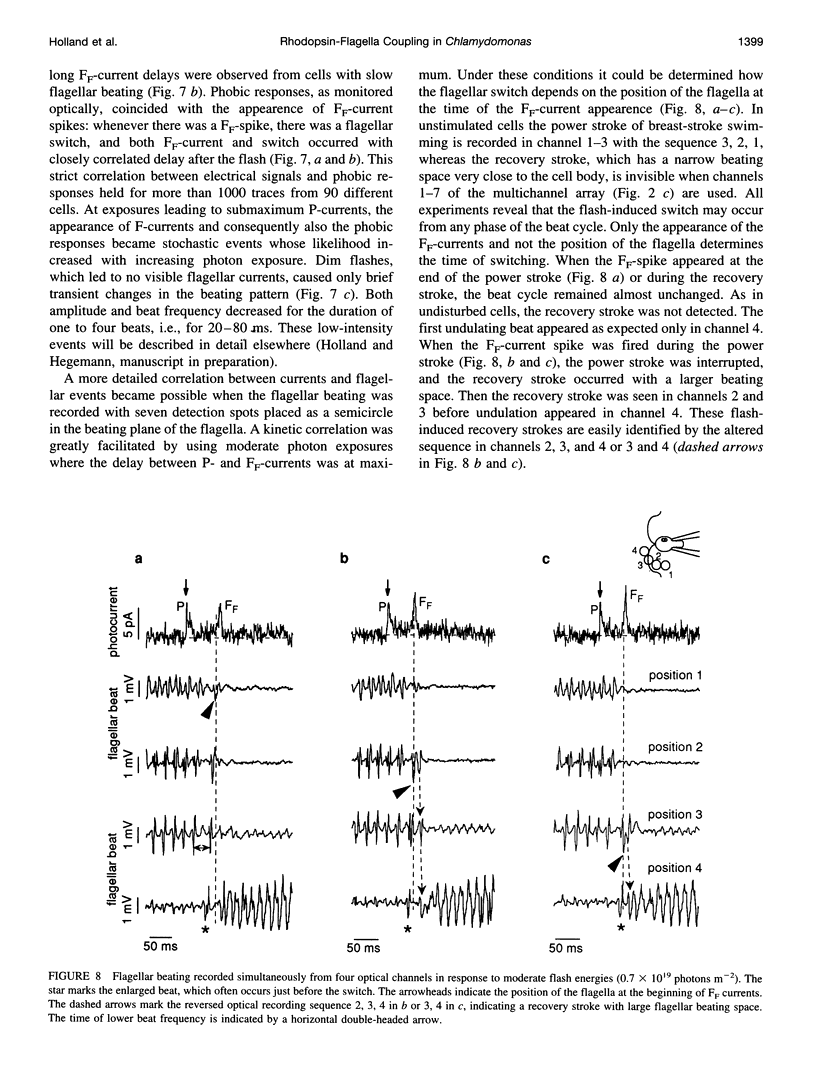

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrivon C. Membrane control of ciliary movement in ciliates. Biol Cell. 1988;63(2):133–142. doi: 10.1016/0248-4900(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Beck C., Uhl R. On the localization of voltage-sensitive calcium channels in the flagella of Chlamydomonas reinhardtii. J Cell Biol. 1994 Jun;125(5):1119–1125. doi: 10.1083/jcb.125.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen M., Fay R. B., Witman G. B. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J Cell Biol. 1980 Aug;86(2):446–455. doi: 10.1083/jcb.86.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. W., Smyth R. D. Light Antennas in phototactic algae. Microbiol Rev. 1980 Dec;44(4):572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E. M., Braun F. J., Nonnengässer C., Harz H., Hegemann P. The nature of rhodopsin-triggered photocurrents in Chlamydomonas. I. Kinetics and influence of divalent ions. Biophys J. 1996 Feb;70(2):924–931. doi: 10.1016/S0006-3495(96)79635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams J. S., Borisy G. G. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J Cell Sci. 1978 Oct;33:235–253. doi: 10.1242/jcs.33.1.235. [DOI] [PubMed] [Google Scholar]
- Kamiya R., Witman G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984 Jan;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra B., Glass ADM. Potassium Fluxes in Chlamydomonas reinhardtii (I.Kinetics and Electrical Potentials). Plant Physiol. 1995 Aug;108(4):1527–1536. doi: 10.1104/pp.108.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto C. K., Brokaw C. J. Bending patterns of Chlamydomonas flagella: II. Calcium effects on reactivated Chlamydomonas flagella. Cell Motil. 1985;5(1):53–60. doi: 10.1002/cm.970050105. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Eckert R. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature. 1976 Aug 19;262(5570):713–715. doi: 10.1038/262713a0. [DOI] [PubMed] [Google Scholar]
- Sineshchekov O. A., Litvin F. F., Keszthelyi L. Two components of photoreceptor potential in phototaxis of the flagellated green alga Haematococcus pluvialis. Biophys J. 1990 Jan;57(1):33–39. doi: 10.1016/S0006-3495(90)82504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]