Abstract
Purpose
Human embryonic lethal abnormal visual-like protein, HuR, belongs to a member of the Hu family of RNA-binding protein and plays a critical role in urinary tumors. The purpose of this review is to summarize the current literature to demonstrate the importance of HuR in urinary tract tumors’ biology and explore the potential role in therapeutic strategies aimed at targeting this molecule in cancer cells.
Methods
The relevant literature from PubMed and Medline databases is reviewed in this article.
Results
Increasing evidence supports that HuR plays a critical role in urinary tumors’ biology because it regulates the expression of many urinary tumors-associated molecules through post-transcriptional regulatory mechanisms (including mRNA trafficking, mRNA decay and protein translation). Recent studies have demonstrated that HuR is associated with chemoresistance of urinary tumors, suggesting that HuR might be a novel therapeutic target and a marker for therapeutic response and prognosis assessment.
Conclusion
HuR is associated with various urinary tumors biological characteristics. Targeted therapy of HuR may become an attractive treatment strategy. What’s more, more preclinical and clinical trials of this targeted strategy are necessary for the treatment of urinary tumors.
Keywords: ELAV1, HuR, Urinary neoplasms, Biological characteristics, Chemotherapy resistance
Introduction
Human antigen R (HuR) belongs to a member of the ELAV family of RNA-binding proteins. HuR is extensively expressed in multiple tumors compared with other ELAV family members (HuB, HuC, and HuD) which are exclusively expressed in neuronal tissues (Robinow et al. 1988). HuR is predominantly localized to the nucleus at resting state. Under a variety of stimulations, HuR can recognize and bind to tumor-associated mRNAs which are enriched with adenine/uridine (AU) or uridine (U) elements through three typical RNA recognition motifs (RRMs). The HuR–mRNA complex is then transferred to the cytoplasm, thereby exerting its function of stabilizing the target mRNA and regulating translation of proteins (von Roretz et al. 2011). Current studies showed that HuR was associated with various tumor biological characteristics, including tumor development and progression, invasion, migration, prognosis and chemotherapy resistance. Totally, HuR plays a key regulatory role in various biological characteristics of tumors by regulating the expression of cancer-associated genes through its post-transcriptional regulatory mechanisms (including mRNA trafficking, mRNA decay, and protein translation).
Urinary tumors predominantly include prostate cancer, bladder cancer, renal pelvis cancer and penile cancer. It is well known that bladder cancer ranks first among urological cancers. As the fourth most common cancer worldwide, bladder cancer with an estimated incidence of > 350,000 new cases per year (Siegel et al. 2013). In addition, prostate cancer (PCa) is one of the most common malignancies in developed countries and is the most frequently diagnosed second leading cause of cancer-related mortality among men. It is estimated that there are 220,800 newly diagnosed cases and more than 29,000 deaths annually in the United States (Siegel et al. 2015; Roychowdhury and Chinnaiyan 2013). Similarly, renal cell carcinoma (RCC) is the most lethal urological cancer and the sixth leading cause of cancer deaths in Western countries and accounts for 3% of adult malignancies and resulting in more than 100,000 deaths worldwide annually (Clark 2007; Sourbier and Massfelder 2006). We and others focused on investigate the key molecular mechanism of post-transcriptional gene regulation that is altered in urinary tumors cells. Specifically, HuR, a molecule extensively expressed in cancers, plays an important role in urinary tumors. In this paper, we comprehensively survey the evidence to the multiple functions of HuR in urinary tumors and explore a new targeted therapy strategy.
Expression of HuR in urinary tumors
Current researches showed that the accumulation of cytoplasmic HuR have been found in pancreatic cancer, colorectal cancer, lung cancer, breast cancer, gastric cancer, oral cancer, liver cancer, glioma and ovarian cancer (Koljonen et al. 2008; Kim et al. 2012; Denkert et al. 2004a, b, 2006; Mrena et al. 2005; Wang et al. 2009). Studies have gradually affirmed that HuR translocation from the nucleus to the cytoplasm is the basis for its function (Brennan and Steitz 2001; Wang et al. 2013). In bladder cancer, renal cancer and prostate cancer, HuR plays a critical regulatory role in biological characteristics by regulating the expression of tumor-associated mRNAs (Fig. 1). Molecular related studies indicate that expression of multiple molecules in urinary tumors is associated with cancer development and progression. In this paper, we conclude that a variety of urinary tumors-associated molecules are targeted by HuR, these molecules are involved in tumorigenesis and development except the tumor suppressor gene p53. Therefore, the function of HuR in tumors may be the result of a balance between tumor suppression and tumor promotion. However, almost all studies have shown that HuR acts as an oncogene in urinary tumors. Therefore, HuR plays an important role in urinary tumors.
Fig. 1.

The multiple functions of HuR in urinary neoplasms’ biological characteristics through the regulation of the expression of multiple cancer-related proteins. COX-2 cyclooxygenase-2, VEGF vascular endothelial growth factor, HO-1 heme oxygenase, TGF-β transforming growth factor-β, IGF-IR insulin-like growth factor I receptor, MMP9 matrix metalloproteinase-9, SIRT1 silent mating type information regulation 2 homolog 1, HIF hypoxia-inducible factor, AR androgen receptor, ABCG2 ATP-binding cassette (ABC) subfamily G member 2, PTHrP parathyroid hormone-related protein, HOTAIR long noncoding HOX transcript antisense RNA, LIG4 DNA ligase IV, BRCA2 breast cancer susceptibility gene
Expression of HuR in prostate cancer
The first study to demonstrate that HuR as an androgen receptor (AR) target in prostate cancer was published in 2002 (Yeap et al. 2002). Since then multiple studies have found that HuR was overexpressed in prostate cancer cell lines as well as in tissue samples, especially cytoplasm. In addition, study (Barbisan et al. 2009) showed that expression of cytoplasmic HuR was significantly increased in 40 patients with atrophy, high-grade prostate intraepithelial neoplasia (PIN) and prostate cancer compared with normal epithelial tissue. Interestingly, Mitsunari et al. (2016) detected that cytoplasmic HuR expression was higher in castration-resistant prostate cancer (CRPC) > hormone-naïve prostate cancer > non-neoplastic cells. Therefore, this result preliminary demonstrated that HuR was associated with the development of prostate cancer.
Expression of HuR in bladder cancer
In bladder cancer, Matsuo et al. (2017) revealed that in non-cancer cells, HuR was mainly located in the nucleus, whereas in cancer cells, in addition to nuclei, HuR expression was detected in the cytoplasm. Furthermore, they observed that the percentage of cytoplasmic HuR-positive cancer cells in cases of muscle invasion was higher than that in cases without muscle invasion. In addition, Fus et al. (2018) found that higher expression of HuR was observed in bladder cancer tissues relative to normal tissues. Similarly, Yu et al. (2017) also found that the expression of HuR in bladder cancer cell lines (J82 and T24 cells) was significantly increased compared with normal bladder epithelial cell line (SV-HUC-1). This is similar to the results of several studies. In addition, animal models research (Miyata et al. 2013) showed that HuR expression in muscle invasive bladder cancer was significantly higher than non-muscle invasive bladder cancer. In summary, the significance of high expression of HuR in bladder cancer lies in its role in promoting tumor progression.
Expression of HuR in renal cell carcinoma
Similarly, in renal cell carcinoma, several studies (Ronkainen et al. 2011; Abdelmohsen et al. 2010) have shown that the expression of HuR was increased in vitro as well as in vivo. Moreover, its expression dramatically in the cytoplasm of pT1 tumor cells was more abundant than in pT3, which also showed the relationship between HuR and the progression of renal cancer (Danilin et al. 2010). Therefore, we can draw a definite conclusion that HuR is overexpression in renal, bladder, and prostate cancers which support the notion that HuR is required for cancer initiation and progression. These results also provided a mechanistic basis for targeted therapy strategies of tumors.
The role of HuR in the biological characteristics of urinary tumors
Angiogenesis and lymphangiogenesis
Tumor tissue has abundant blood supply and tumor cells promote angiogenesis through various mechanisms. In addition, tumor angiogenesis has a certain degree of tissue invasiveness performed that tumor cells can invade along the collagen fissure opened by the neovascularization. A variety of molecular regulation mechanisms are involved in this process, including vascular endothelial growth factor A (VEGF-A), vascular endothelial growth factor C(VEGF-C) (Carmeliet 2005), cyclooxygenase-2 (COX-2) (Sahin et al. 2009), hypoxia-inducible factor-α (HIF-α) (Wenger et al. 2005), interleukin-8 (IL-8) (Yoo et al. 2006)and so on. In recent years, tumor-related studies have shown that post-transcriptional regulatory mechanisms play an important role in regulating the expression of these molecules. In bladder cancer, studies have reported that VEGF-A and -C are significantly associated with tumor microvessel density (MVD) and lymphatic vessel density (LVD). Miyata et al. showed that HuR was closely related to bladder cancer MVD and LVD by upregulating the expression of VEGF-C, VEGF-A and COX-2. Matsuo et al. found that green tea polyphenols (GTP) inhibited bladder cancer cell proliferation, angiogenesis and expression of cancer-related factors VEGF-A, COX-2 and cyclooxygenase-1 (HO-1) through direct and indirect regulation mechanisms of HuR. In kidney cancer, several studies (Basu et al. 2010; Xin et al. 2012; Dufies et al. 2017; Datta et al. 2005) have shown that HuR-mediated angiogenesis and cancer progression by modulating the expression of VEGF and COX-2 in renal cancer cells. Moreover, HuR has been shown to regulate the expression of HIF-1α and HIF-2α by targeting mRNA. As known to all, HIF is a global regulator of hypoxia response and participates in the regulation of tumor angiogenesis, proliferation, metastasis and invasion. Therefore, under hypoxic conditions, HuR mediates tumor angiogenesis and development of kidney cancer by modulating the expression of HIF. The interaction between HuR and the above molecules was also observed in prostate cancer. In addition, tumor-producing transforming growth factor (TGF-β1) induces expression of VEGF at both the transcriptional and post-transcriptional levels through multiple pathways including Smad3, HIF-2a and HuR (Chae et al. 2011). Therefore, the production of autocrine TGF-β1 can promote tumor angiogenesis through HIF-2α signal transduction under non-hypoxic conditions, thus providing selective growth advantage for prostate tumor cells. Totally, accumulation of cytoplasmic HuR in urinary tumors involves in angiogenesis or lymphangiogenesis. Efficacy of targeting HuR may inhibit the occurrence and development of tumors. Currently, there are two strategies to target HuR, including small interfering RNA transfection and small molecules inhibition. Knockdown of HuR through siRNA transfecting inhibits urinary tumors cell migration, invasion, proliferation, apoptosis and epithelial–mesenchymal transition process. Furthermore, knockdown of HuR through siRNA transfecting enhances docetaxel cytotoxicity in prostate cancer cells. Moreover, pyrvinium pamoate, as a novel HuR inhibitor dose-dependently inhibits cytoplasmic accumulation of HuR. Combining pyrvinium pamoate with chemotherapeutic agents not only led to enhanced cytotoxicity in bladder cancer cells, but also synergistically suppressed the growth of patient-derived bladder tumor xenografts in mice. In addition, other HuR inhibitors include MS-444, CMLD-2, DHTS. In future, more sophisticated studies will address the safety and effectiveness of targeted HuR treatment strategies in clinical applications.
Invasion and metastasis
Urinary tumor cells have the ability to invade adjacent tissues and proliferate in distant organs. For example, prostatic cancer most commonly metastasizes to lymph nodes and bone. Bladder cancer tends to invade the peripheral pelvic tissues. HuR is also associated with invasion and migration of urinary tumors. Clinical data showed that accumulation of cytoplasmic HuR was significantly associated with lymph node metastasis, invasion, infiltration of blood vessels and nerves and the expression of cyclin A in upper urothelial carcinoma (Liang et al. 2012). The mechanism may be involved in the post-transcriptional regulation of multiple invasive-related molecules including cyclin A. Animal model tests showed that accumulation of cytoplasmic HuR was associated with increased muscle invasion of bladder cancer in mice. Similarly, green tea polyphenols can directly and indirectly regulate HuR to inhibit bladder cancer cell proliferation, angiogenesis and expression of the cancer-related factors VEGF-A, COX-2 and HO-1. In addition, Yu et al. demonstrated for the first time that HuR and HOTAIR, long-chain non-coding RNAs, function in coordination with each other. On the one hand, HuR increases HOTAIR expression by directly binding to HOTAIR in bladder cancer. On the other hand, HOTAIR promotes accumulation of cytoplasmic HuR. Knockdown of HuR or HOTAIR can inhibit bladder cancer cell migration, invasion, proliferation, apoptosis and epithelial-mesenchymal transition. Therefore, the role of HuR in the proliferation, invasion and metastasis of bladder cancer was demonstrated both at the cellular level and animal model tests. Similarly, Danilin et al. (2009, 2010) found that knockdown of HuR could inhibit the proliferation and increase apoptosis which mediate the progression of renal cancer by inhibiting the PI3K/Akt and MAPK oncogenic signaling pathways. Mitsunari et al. (2016) demonstrated that HuR played a crucial role in the aggressiveness and prognosis of PCa, especially androgen-dependent PCa, by regulating cell proliferation, migration, and expression of VEGF-A, -C and COX-2. In addition, progression-associated molecular matrix metalloproteinase-9 (MMP-9) was also found to be regulated by HuR in prostate cancer. Moreover, PCK3145 is a synthetic peptide corresponding to amino acids 31–45 of prostate secretory protein 94, which can reduce bone metastasis and prostate cancer growth, while PCK3145 can inhibit the development of prostate cancer by reducing the expression of HuR and subsequently reducing the expression of MMP-9 (Annabi et al. 2006). In conclusion, HuR-mediated cancer progression follows the upregulation of HuR-targeted mRNAs encoding proteins that alter the aggressive potential of cancer cells. However, detailed molecular mechanisms still beyond study.
Clinical pathological features
Current studies have demonstrated that the status of HuR in human malignancies was significantly related to its expression in normal tissues and pre-malignant lesions. For example, comparison of HPV-induced low-grade and high-grade pre-malignant lesions with cervical cancer shows increased expression of HuR in tumor lesions and highest expression in cervical cancer (Fay et al. 2009). These findings insinuate the nucleocytoplasmic translocation and cytoplasmic presence of HuR is necessary for its function in various carcinomas although the mechanism remains unclear. HuR is upregulated in tumor tissues and its nucleocytoplasmic transfer plays a key role in stabilizing targeted mRNAs. By contrast, there are few cells with a positive expression of cytoplasmic HuR in normal tissues. Stromal cells and adjacent non-neoplastic tissue do not show cytoplasmic expression of HuR. In non-cancer cells, HuR immunoreactivity is mostly observed in the nuclei, it suggests that HuR might be at resting status in normal cells. HuR is also significantly associated with the tumor stage, grade, and clinicopathological features of urothelial tumors. Clinical data showed that there was a significant correlation between cytoplasmic HuR-positive and high tumor grade and pT stage of bladder cancer, indicating that HuR is involved in obtaining malignant histopathological features and the ability to invade the muscularis propria. However, there was no correlation between nuclear HuR expression. In addition, Liang et al. showed that the high expression of cyclin A was associated with higher pT stage, high histological grade and frequent mitosis of bladder cancer cells. Furthermore, cyclin A was also upregulated by HuR. Similarly, the accumulation of cytoplasmic HuR is associated with age, clinical stage, Fuhrman grade and prognosis of kidney cancer (Ronkainen et al. 2011; Barbisan et al. 2009). In addition, the correlation of COX-2 was also observed. Therefore, cytoplasmic HuR expression is associated with reduced RCC-specific survival. In prostate cancer, HuR cytoplasmic accumulation is positively correlated with Gleason score, T stage and metastasis, and it is considered to be a useful predictor of biochemical recurrence after radical prostatectomy. Interestingly, expression of cytoplasmic HuR is highest in CRPC. We speculate that HuR is also responsible for the progression of hormone-sensitive prostate cancer to CRPC, and its function is closely related to the regulation of the expression of VEGF-A, VEGF-C and COX-2. Therefore, HuR plays an extremely important role in the malignant progression of urinary tumors, its mRNA-stabilizing function is essential for the development of cancer although the molecular mechanism remains to be further studied.
HuR is towardly to become a new biomarker in urinary tumors
HuR is abundant in majority cancers and reduced in normal cells, and the cytoplasmic HuR status in tumor specimens have been shown to associate with poor prognostic value in many tumor types, including pancreatic cancer, colorectal cancer, lung cancer, gastric cancer, breast cancer, and ovarian cancer (Heinonen et al. 2005; Lim et al. 2007; Kurosu et al. 2011; Cha et al. 2011; Zhu et al. 2013). HuR is predominantly localized within the nucleus of resting status and can shuttle between the nucleus and the cytoplasm. The ability of HuR to promote mRNA stabilization requires its translocation to the cytoplasm. In the context of cancer, HuR as an mRNA stability factor in response to various cancer-associated stressors, including ultraviolet radiation (UVC), lipopolysaccharide (LPS), chemical compounds, alterations in the microenvironment, cytokines, viral infection and hormone treatment, where it binds to ARE-containing mRNAs in the nucleus and then HuR–mRNA complex transported to the cytoplasm. Nucleocytoplasmic trafficking is mediated through a basic 32-amino acid HuR nucleocytoplasmic shuttling (HNS) sequence contained in the hinge region and involves several transport machinery components including exportin-1 (XPO1, CRM1), transportins, and importins. However, molecular mechanism researches still need further study. In most of the published retrospective studies, immunohistochemistry was used to evaluate the intracellular expression pattern of HuR in human malignancies. It was found that HuR cytoplasmic expression was associated with poor patient survival, disease-free survival, metastasis-free survival, or overall survival. Miyata et al. found that cytoplasmic HuR expression was significantly related to the malignant potential, tumor progression and prognosis of bladder cancer. On the contrary, there was no association between nuclear expression of HuR and prognosis. Similarly, Ronkainen et al. also found that cytoplasmic HuR expression was associated with decreased RCC-specific survival, which is the same as that of most tumors. However, studies of patients with pancreatic cancer that received potentially curative pancreatectomy have shown that HuR cytoplasmic accumulation was a predictive marker for gemcitabine sensitivity and good prognosis (Kurosu et al. 2011). Miyata et al. (2017) found that there was no correlation between nuclear HuR expression levels and the overall survival of patients with advanced urothelial carcinoma (UC), and cytoplasmic HuR expression was an important positive predictor of chemotherapy response based on gemcitabine in patients with cisplatin-resistant UC. In addition, Niesporek et al. (2008) found that nuclear HuR expression was an advantageous prognostic factor for the disease-free survival of prostate cancer. We speculate that these differences may be due to the inclusion of different stage patients and disruption of HuR’s biology by different therapeutic regimens. In summary, a significant correlation between HuR and tumor T stage has been shown, and cytoplasmic HuR expression is also observed as a marker of poor prognosis in most tumors. Therefore, HuR may become a significant biomarker of urinary tumor. Unfortunately, previous reviews were based on retrospective studies, while validation of HuR as a predictor marker should be performed in a prospective manner and focus on clinical trials with advanced or metastatic patients.
HuR is a vital modulator of urinary tumors chemoresistance
Nucleocytoplasmic translocation and cytoplasmic presence of HuR not only affects the biological characteristics of tumors. In recent years, HuR has been implicated in inducing chemoresistance. Among a majority of cancers include pancreatic cancer, colorectal cancer, lung cancer, breast cancer, oral cancer and glioma (Latorre et al. 2012, 2014; Zhou et al. 2016; To et al. 2015; Lin et al. 2017; Janakiraman et al. 2017; Filippova et al. 2011), silencing of HuR by small interfering RNA transfecting or small molecules inhibiting can promote the sensitivity of tumor cells to chemotherapy drugs. Many possibilities could account for why HuR status correlates with chemoresistance is that HuR not only regulates the expression of a variety of tumor drug-resistance-related molecules, but also changes the tumor microenvironment, thereby affecting the sensitivity of tumor treatment.
HuR and prostate cancer chemotherapy resistance
Prostate cancer is the second leading cause of cancer-related mortality among men in developed countries. Androgen deprivation therapy (ATD) is the main initial systemic treatment for metastatic hormone-sensitive prostate cancer (Long et al. 2000). Although ADT initially led to a 2–3 year remission, most patients eventually relapse with castration-resistant prostate cancer (CRPC) (Ning et al. 2016). For patients with CRPC, docetaxel (DTX)-based chemotherapy has become the standard of first-line therapy for prolonging survival and improving quality of life (Nakano et al. 2016). However, the development of DTX resistance remains a challenge in prostate cancer treatment.
In prostate cancer, there is downregulation of miR-133b and upregulation of HuR. miR-133b can target HuR and inhibit its expression. Ectopic expression of miR-133b and HuR knockdown inhibits cell viability and promotes DTX-induced apoptosis in DTX-treated prostate cancer cells, whereas overexpression of HuR reverses this effect. In addition, both miR-133b and HuR can target the ATP-binding cassette (ABC) subfamily G member 2 (ABCG2), and overexpression of HuR partially eliminate the inhibitory effect of miR-133b on ABCG2 expression (Liu et al. 2018). It is well known that ABCG2 relies on ATP hydrolysis to pump chemotherapeutic drugs out of the cell to reduce intracellular drug concentration and drug toxicity. It is one of the potential mechanisms of multidrug resistance in tumors (Zhang et al. 2016). In conclusion, post-transcriptional regulation of HuR by miR-133b enhances DTX cytotoxicity by inhibiting ABCG2, revealing a novel miR-133b/HuR/ABCG2 regulatory pathway to reverse chemotherapy resistance in prostate cancer (Fig. 2). In addition, Kojima et al. (2010) found that in prostate cancer cells shows down-regulation of miR-34a and up-regulation of HuR, miR-34a acts on the 3′-UTR of SIRT1 and Bcl2 mRNAs via regulating HuR expression directly or indirectly (Fujita et al. 2008), thereby controlling their expression (Fig. 3). SIRT1 (silent mating type information regulator 2 homologue 1) is a NAD+ dependent histone deacetylase that deacetylates histones and some non-histone proteins such as p53. SIRT1 plays a key role in various cellular processes including cell survival under oxidative stress and genotoxicity. SIRT1 is upregulated in great majority cancers including prostate cancer and is associated with tumor development and chemoresistance (Jung-Hynes et al. 2009). Bcl-2 is a major anti-apoptotic factor and is also one of the important mechanisms of multidrug resistance in tumors (Wang et al. 2012). In addition, Epis (2011) found that the expression of ERBB-2 in prostate cancer can be regulated by HuR. It is well known that ERBB-2 overexpression is significantly associated with the development and progression of cancers and chemotherapy resistance, which also provides a theoretical basis for the study of molecular mechanisms. Therefore, miR-34a and HuR play an important role in the development of paclitaxel resistance and may become a useful biomarker and promising therapeutic target for drug resistance in hormone-refractory prostate cancer.
Fig. 2.
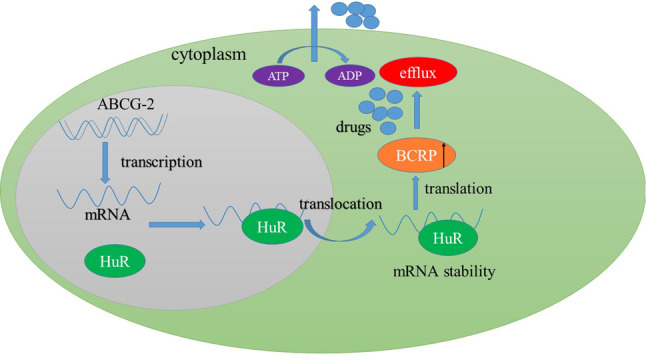
In prostate cancer cells, HuR can bind to target mRNA and then exported from the nucleus to the cytoplasm, where it stabilizes the target mRNAs. Once expression of BCRP protein is upregulated by HuR, the efflux of chemotherapy drugs will increase, thus result in chemoresistance
Fig. 3.
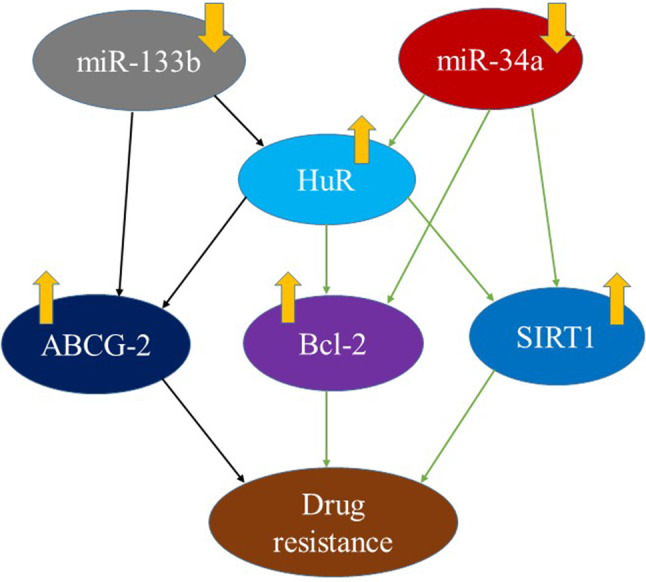
In prostate cancer, miR133b mediates the drug resistance through regulating the expression of HuR and ABCG2; miR34a mediates the drug resistance through regulating the expression of HuR, bcl-2 and SIRT1
HuR and bladder cancer chemotherapy resistance
Platinum-based systemic chemotherapy is currently the standard treatment for patients with bladder urothelial carcinoma (UCB) (Gupta and Mahipal 2013). Unfortunately, approximately half of UCB patients are not suitable for cisplatin treatment due to poor response or unbearable renal failure (Cathomas et al. 2015; Maeda et al. 2007). Therefore, finding a solution to increase the sensitivity of chemotherapy is paramount importance. pyrvinium pamoate, a novel HuR small molecule inhibitor, promotes nuclear import of HuR by activating the AMP-activated kinase/importin α1 cascade and impedes HuR nucleocytoplasmic translocation by inhibiting the checkpoint kinase1/cyclin-dependent kinase 1 pathway, which in turn reduces cytoplasmic accumulation of HuR. Furthermore, pyrvinium pamoate significantly downregulates several major DNA repair genes, including DNA ligase IV and BRCA2, thereby reducing DNA repair and leading to genomic instability and cell death (Guo et al. 2016). In addition, pyrvinium pamoate significantly contributed to a durable cytostatic tumor growth response when combined with cisplatin in preclinical bladder cancer mouse models. Interestingly, mice bearing primary bladder cancer xenografts did not lose weight or suffer other significant side effects at tested dosages, which demonstrated the safety of HuR. Combination of HuR inhibitors and DNA damage chemotherapy drugs can reverse multidrug resistance in tumors. Targeted therapy strategies for HuR will open a new era in drug treatment.
Conclusion
Under various stimulations, the HuR protein has the ability to translocate from the nucleus to the cytoplasm, thereby stabilizing the target mRNA. Post-transcriptional modifications appear to control the abundance, localization and binding of HuR to the mRNA. Thus, inhibition of HuR cytoplasm accumulation with the administration of current therapeutic agents may lead to successful treatment strategies. With the development of cancer-related research, HuR is becoming an attractive target for urologic tumors and other tumors. For example, HuR has been proved to be a molecular marker of malignant tumors and plays an oncogenic role in various tumors. However, the concept of HuR still needs further exploration. For example, what is the number of specific HuR target mRNAs in urinary tumors? Which are the most critical HuR-target mRNAs in urinary tumors? And by which molecules HuR is regulated? How long HuR can promote survival effects? These interesting questions leave plenty of space for future research. Although we focused on the role of HuR in the post-transcriptional regulation of various transcripts, we also found that the regulation of its own function and expression remains complicated. For instance, HuR mRNA is regulated by Smad, TTP, RNP C1, Mdm2, pp32 and Hsf1. In addition, many miRNAs negatively regulate the expression of HuR through interacting with 3′UTR and 5′UTR of the HuR mRNA, including miR-34a, miR-133b, miR-200, miR-519, miR-9. Therefore, the adjustment mechanism of HuR might be a complex network system. With the continuous deepening of the research on molecular mechanisms, preclinical animal model experiments and large-scale clinical trials have yet to be carried out. These efforts will address the safety and practicality of HuR-targeted therapy. With the deepening of HuR research, we firmly believe that HuR will open a new chapter in urinary tumors and tumor-targeted therapeutic strategies.
Funding
This study was funded by the Talent Innovation and Enterprise Program of Lanzhou (Grant no. 2015-RC-16).
Compliance with ethical standards
Conflict of interest
Authors have no conflicts of interest to declare.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Fa Zhang, Zhonglin Cai and Haidi Lv contributed equally.
References
- Abdelmohsen K, Kim MM, Srikantan S (2010) miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle 9(7):1354–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi B, Bouzeghrane M, Currie JC (2006) Inhibition of MMP-9 secretion by the anti-metastatic PSP94-derived peptide PCK3145 requires cell surface laminin receptor signaling. Anticancer Drugs 17(4):429–438 [DOI] [PubMed] [Google Scholar]
- Barbisan F, Mazzucchelli R, Santinelli A (2009) Overexpression of ELAV-like protein HuR is associated with increased COX-2 expression in atrophy, high-grade prostatic intraepithelial neoplasia, and incidental prostate cancer in cystoprostatectomies. Eur Urol 56(1):105–112 [DOI] [PubMed] [Google Scholar]
- Basu A, Datta D, Zurakowski D (2010) Altered VEGF mRNA stability following treatments with immunosuppressive agents: implications for cancer development. J Biol Chem 13(33):25196–25202 285( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CM, Steitz JA (2001) HuR and mRNA stability. Cell Mol Life Sci 58(2):266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10 [DOI] [PubMed] [Google Scholar]
- Cathomas R, De Santis M, Galsky MD (2015) First-line treatment of metastatic disease: cisplatin-ineligible patients. Hematol Oncol Clin N Am 29(2):329–340, x [DOI] [PubMed] [Google Scholar]
- Cha JD, Li S, Cha IH (2011) Association between expression of embryonic lethal abnormal vision-like protein HuR and cyclooxygenase-2 in oral squamous cell carcinoma. Head Neck 33(5):627–637 [DOI] [PubMed] [Google Scholar]
- Chae KS, Kang MJ, Lee JH (2011) Opposite functions of HIF-α isoforms in VEGF induction by TGF-β1 under non-hypoxic conditions. Oncogene 30(10):1213–1228 [DOI] [PubMed] [Google Scholar]
- Clark PE (2007) Recent advances in targeted therapy for renal cell carcinoma. Curr Opin Urol 17(5):331–336 [DOI] [PubMed] [Google Scholar]
- Danilin S, Sourbier C, Thomas L (2009) von Hippel-Lindau tumor suppressor gene-dependent mRNA stabilization of the survival factor parathyroid hormone-related protein in human renal cell carcinoma by the RNA-binding protein HuR. Carcinogenesis 30(3):387–396 [DOI] [PubMed] [Google Scholar]
- Danilin S, Sourbier C, Thomas L (2010) Role of the RNA-binding protein HuR in human renal cell carcinoma. Carcinogenesis 31(6):1018–1026 [DOI] [PubMed] [Google Scholar]
- Datta K, Mondal S, Sinha S (2005) Role of elongin-binding domain of von Hippel Lindau gene product on HuR-mediated VPF/VEGF mRNA stability in renal cell carcinoma. Oncogene 24(53):7850–7858 [DOI] [PubMed] [Google Scholar]
- Denkert C, Weichert W, Winzer KJ (2004a) Expression of the ELAV-like protein HuR is associated with higher tumor grade and increased cyclooxygenase-2 expression in human breast carcinoma. Clin Cancer Res 10(16):5580–5586 [DOI] [PubMed] [Google Scholar]
- Denkert C, Weichert W, Pest S (2004b) Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Res 64(1):189–195 [DOI] [PubMed] [Google Scholar]
- Denkert C, Koch I, von Keyserlingk N et al (2006) Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol 19(9):1261–1269 [DOI] [PubMed] [Google Scholar]
- Dufies M, Giuliano S, Ambrosetti D (2017) Sunitinib stimulates expression of VEGFC by tumor cells and promotes lymphangiogenesis in clear cell renal cell carcinomas. Cancer Res 77(5):1212–1226 [DOI] [PubMed] [Google Scholar]
- Epis MR, Barker A, Giles KM (2011) The RNA-binding protein HuR opposes the repression of ERBB-2 gene expression by microRNA miR-331-3p in prostate cancer cells. J Biol Chem 286(48):41442–41454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay J, Kelehan P, Lambkin H (2009) Increased expression of cellular RNA-binding proteins in HPV-induced neoplasia and cervical cancer. J Med Virol 81(5):897–907 [DOI] [PubMed] [Google Scholar]
- Filippova N, Yang X, Wang Y et al (2011) The RNA-binding protein HuR promotes glioma growth and treatment resistance. Mol Cancer Res 9(5):648–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M (2008) Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun 377(1):114–119 [DOI] [PubMed] [Google Scholar]
- Fus ŁP, Pihowicz P, Koperski Ł (2018) High cytoplasmic HuR expression is associated with advanced pT stage, high grade and increased microvessel density in urothelial bladder carcinoma. Ann Diagn Pathol 33:40–44 [DOI] [PubMed] [Google Scholar]
- Guo J, Lv J, Chang S (2016) Inhibiting cytoplasmic accumulation of HuR synergizes genotoxic agents in urothelial carcinoma of the bladder. Oncotarget 7(29):45249–45262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Mahipal A (2013) Role of systemic chemotherapy in urothelial urinary bladder cancer. Cancer Control 20(3):200–210 [DOI] [PubMed] [Google Scholar]
- Heinonen M, Bono P, Narko K (2005) Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res 65(6):2157–2161 [DOI] [PubMed] [Google Scholar]
- Janakiraman H, House RP, Talwar S et al (2017) Repression of caspase-3 and RNA-binding protein HuR cleavage by cyclooxygenase-2 promotes drug resistance in oral squamous cell carcinoma. Oncogene 36(22):3137–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Hynes B, Nihal M, Zhong W, Ahmad N (2009) Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J Biol Chem 284(6):3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Li S, Cha JD et al (2012) Significance of molecular markers in survival prediction of oral squamous cell carcinoma. Head Neck 34(7):929–936 [DOI] [PubMed] [Google Scholar]
- Kojima K, Fujita Y, Nozawa Y et al (2010) MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate 70(14):1501–1512 [DOI] [PubMed] [Google Scholar]
- Koljonen V, Böhling T, Haglund C (2008) Expression of HuR in Merkel cell carcinoma and in normal skin. J Cutan Pathol 35(1):10–14 [DOI] [PubMed] [Google Scholar]
- Kurosu T, Ohga N, Hida Y (2011) HuR keeps an angiogenic switch on by stabilising mRNA of VEGF and COX-2 in tumour endothelium. Br J Cancer 104(5):819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E, Tebaldi T, Viero G et al (2012) Downregulation of HuR as a new mechanism of doxorubicin resistance in breast cancer cells. Mol Cancer 11:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E, Castiglioni I, Gatto P et al (2014) Loss of protein kinase Cδ/HuR interaction is necessary to doxorubicin resistance in breast cancer cell lines. J Pharmacol Exp Ther 349(1):99–106 [DOI] [PubMed] [Google Scholar]
- Liang PI, Li WM, Wang YH (2012) HuR cytoplasmic expression is associated with increased cyclin A expression and poor outcome with upper urinary tract urothelial carcinoma. BMC Cancer 12:611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SJ, Kim HJ, Kim JY (2007) Expression of HuR is associated with increased cyclooxygenase-2 expression in uterine cervical carcinoma. Int J Gynecol Pathol 26(3):229–234 [DOI] [PubMed] [Google Scholar]
- Lin GL, Ting HJ, Tseng TC et al (2017) Modulation of the mRNA-binding protein HuR as a novel reversal mechanism of epirubicin-triggered multidrug resistance in colorectal cancer cells. PLoS ONE 12(10):e0185625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Song X, Hou J et al (2018) Posttranscriptional regulation of human antigen R by miR-133b enhances docetaxel cytotoxicity through the inhibition of ATP-binding cassette subfamily G member 2 in prostate cancer cells. DNA Cell Biol 37(3):210–219 [DOI] [PubMed] [Google Scholar]
- Long BJ, Grigoryev DN, Nnane IP (2000) Antiandrogenic effects of novel androgen synthesis inhibitors on hormone-dependent prostate cancer. Cancer Res 60(23):6630–6640 [PubMed] [Google Scholar]
- Maeda T, Takahashi A, Hirobe M (2007) Adverse events of MVAC chemotherapy in patients with advanced urothelial cancer of the bladder. Hinyokika Kiyo 53(4):213–219 [PubMed] [Google Scholar]
- Matsuo T, Miyata Y, Asai A (2017) Green tea polyphenol induces changes in cancer-related factors in an animal model of bladder cancer. PLoS One 12(1):e0171091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunari K, Miyata Y, Asai A (2016) Human antigen R is positively associated with malignant aggressiveness via upregulation of cell proliferation, migration, and vascular endothelial growth factors and cyclooxygenase-2 in prostate cancer. Transl Res 175:116–128 [DOI] [PubMed] [Google Scholar]
- Miyata Y, Watanabe S, Sagara Y (2013) High expression of HuR in cytoplasm, but not nuclei, is associated with malignant aggressiveness and prognosis in bladder cancer. PLoS One 8(3):e59095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Mitsunari K, Akihiro A (2017) Human antigen R as a predictive marker for response to gemcitabine-based chemotherapy in advanced cisplatin-resistant urothelial cancer. Oncol Lett 13(2):811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrena J, Wiksten JP, Thiel A et al (2005) Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin Cancer Res 11(20):7362–7368 [DOI] [PubMed] [Google Scholar]
- Nakano M, Shoji S, Higure T (2016) Low-dose docetaxel, estramustine and prednisolone combination chemotherapy for castration-resistant prostate cancer. Mol Clin Oncol 4(6):942–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesporek S, Kristiansen G, Thoma A (2008) Expression of the ELAV-like protein HuR in human prostate carcinoma is an indicator of disease relapse and linked to COX-2 expression. Int J Oncol 32(2):341–347 [PubMed] [Google Scholar]
- Ning P, Zhong JG2, Jiang F (2016) Role of protein S in castration-resistant prostate cancer-like cells. Endocr Relat Cancer 23(8):595–607 [DOI] [PubMed] [Google Scholar]
- Robinow S, Campos AR, Yao KM (1988) The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science 242(4885):1570–1572 [DOI] [PubMed] [Google Scholar]
- Ronkainen H, Vaarala MH, Hirvikoski P, Ristimäki A (2011) HuR expression is a marker of poor prognosis in renal cell carcinoma. Tumour Biol 32(3):481–487 [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, Chinnaiyan AM (2013) Advancing precision medicine for prostate cancer through genomics. J Clin Oncol 31(15):1866–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, Sahin E, Gümüslü S (2009) Cyclooxygenase-2 in cancer and angiogenesis. Angiology 60(2):242–253 [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics (2013) CA Cancer J Clin 63(1):11–30 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics (2015) CA Cancer J Clin 65(1):5–29 [DOI] [PubMed] [Google Scholar]
- Sourbier C, Massfelder T (2006) Parathyroid hormone-related protein in human renal cell carcinoma. Cancer Lett 240(2):170–182 [DOI] [PubMed] [Google Scholar]
- To KK, Leung WW, Ng SS (2015) Exploiting a novel miR-519c-HuR-ABCG2 regulatory pathway to overcome chemoresistance in colorectal cancer. Exp Cell Res 338(2):222–231 [DOI] [PubMed] [Google Scholar]
- von Roretz C, Di Marco S, Mazroui R (2011) Turnover of AU-rich-containing mRNAs during stress: a matter of survival. Wiley Interdiscip Rev RNA 2(3):336–347 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao W, Guo Y et al (2009) The expression of RNA-binding protein HuR in non-small cell lung cancer correlates with vascular endothelial growth factor-C expression and lymph node metastasis. Oncology 76(6):420–429 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang X, Zhao H et al (2012) Clusterin confers resistance to TNF -alpha -induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J Chemotherapy 24(24):348–357 [DOI] [PubMed] [Google Scholar]
- Wang J, Guo Y, Chu H et al (2013) Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int J Mol Sci 14(5):10015–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger RH, Stiehl DP, Camenisch G (2005) Integration of oxygen signaling at the consensus HRE. Sci STKE 2005(306):re12 [DOI] [PubMed] [Google Scholar]
- Xin H, Brown JA, Gong C (2012) Association of the von Hippel-Lindau protein with AUF1 and posttranscriptional regulation of VEGFA mRNA. Mol Cancer Res 10(1):108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap BB, Voon DC, Vivian JP (2002) Novel binding of HuR and poly(C)-binding protein to a conserved UC-rich motif within the 3′-untranslated region of the androgen receptor messenger RNA. J Biol Chem 277(30):27183–27192 [DOI] [PubMed] [Google Scholar]
- Yoo PS, Mulkeen AL, Cha CH (2006) Post-transcriptional regulation of vascular endothelial growth factor: implications for tumor angiogenesis. World J Gastroenterol 12(31):4937–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Zhang C, Gui J (2017) RNA-binding protein HuR promotes bladder cancer progression by competitively binding to the long noncoding HOTAIR with miR-1. Onco Targets Ther 10:2609–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Zhang GN, Wang YJ et al (2016) Bafetinib (INNO-406) reverses multidrug resistance by inhibiting the efflux function of ABCB1 and ABCG2 transporters. Sci Rep 6:25694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chang R, Ji W et al (2016) Loss of scribble promotes snail translation through translocation of HuR and enhances cancer drug resistance. J Biol Chem 291(1):291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Wang B, Bi J (2013) Cytoplasmic HuR expression correlates with P-gp, HER-2 positivity, and poor outcome in breast cancer. Tumour Biol 34(4):2299–2308 [DOI] [PubMed] [Google Scholar]


