Abstract
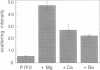
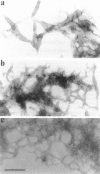
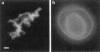
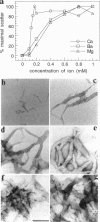
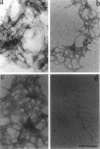
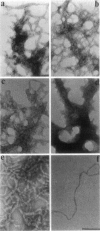
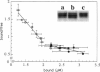
Phosphatidylinositol bisphosphate (PIP2) serves as a precursor for diacylglycerol and inositol trisphosphate in signal transduction cascades and regulates the activities of several actin binding proteins that influence the organization of the actin cytoskeleton. Molecules of PIP2 form 6-nm diameter micelles in water, but aggregate into larger, multilamellar structures in physiological concentrations of divalent cations. Electron microscopic analysis of these aggregates reveals that they are clusters of striated filaments, suggesting that PIP2 aggregates form stacks of discoid micelles rather than multilamellar vesicles or inverted hexagonal arrays as previously inferred from indirect observations. The distance between striations within the filaments varies from 4.2 to 5.4 nm and the diameter of the filaments depends on the dehydrated ionic radius of the divalent cation, with average diameters of 19, 12, and 10 nm for filaments formed by Mg2+, Ca2+, and Ba2+, respectively. The structure of the divalent cation-induced aggregates can be altered by PIP2 binding proteins. Gelsolin and the microtubule associated protein tau both affect the formation of aggregates, indicating that tau acts as a PIP2 binding protein in a manner similar to gelsolin. In contrast, another PIP2 binding protein, profilin, does not modify the aggregates.
Full text
PDF

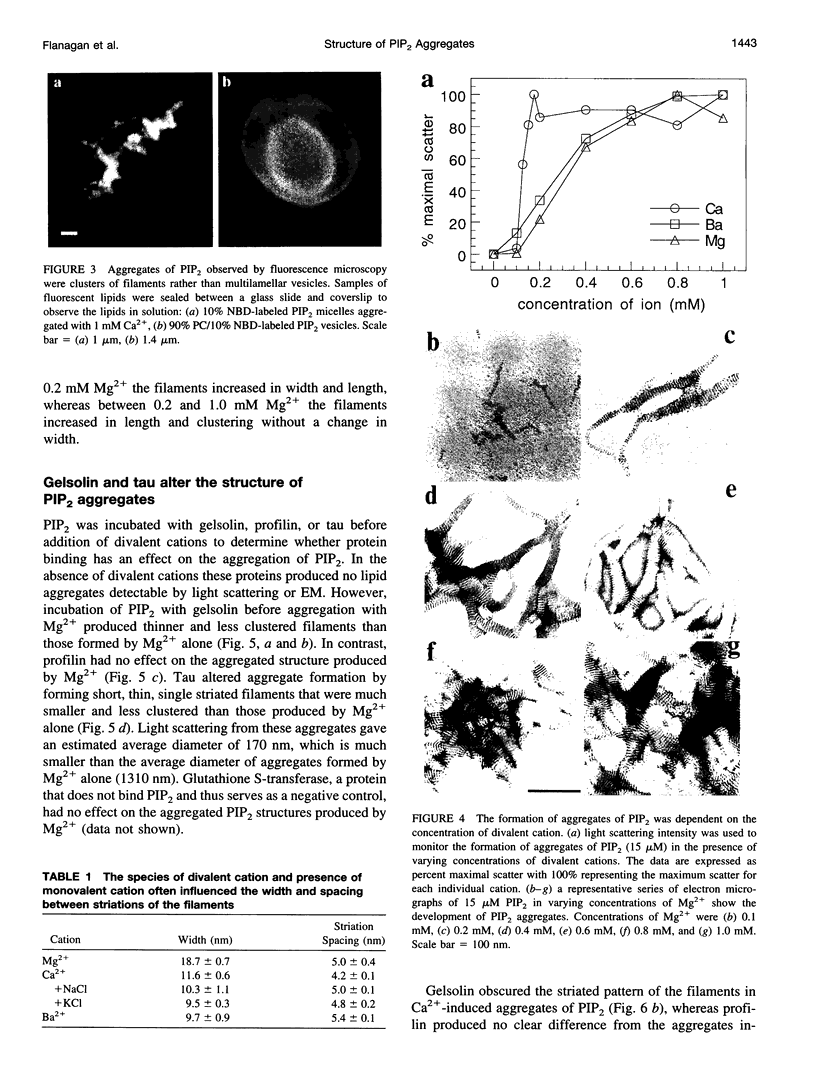
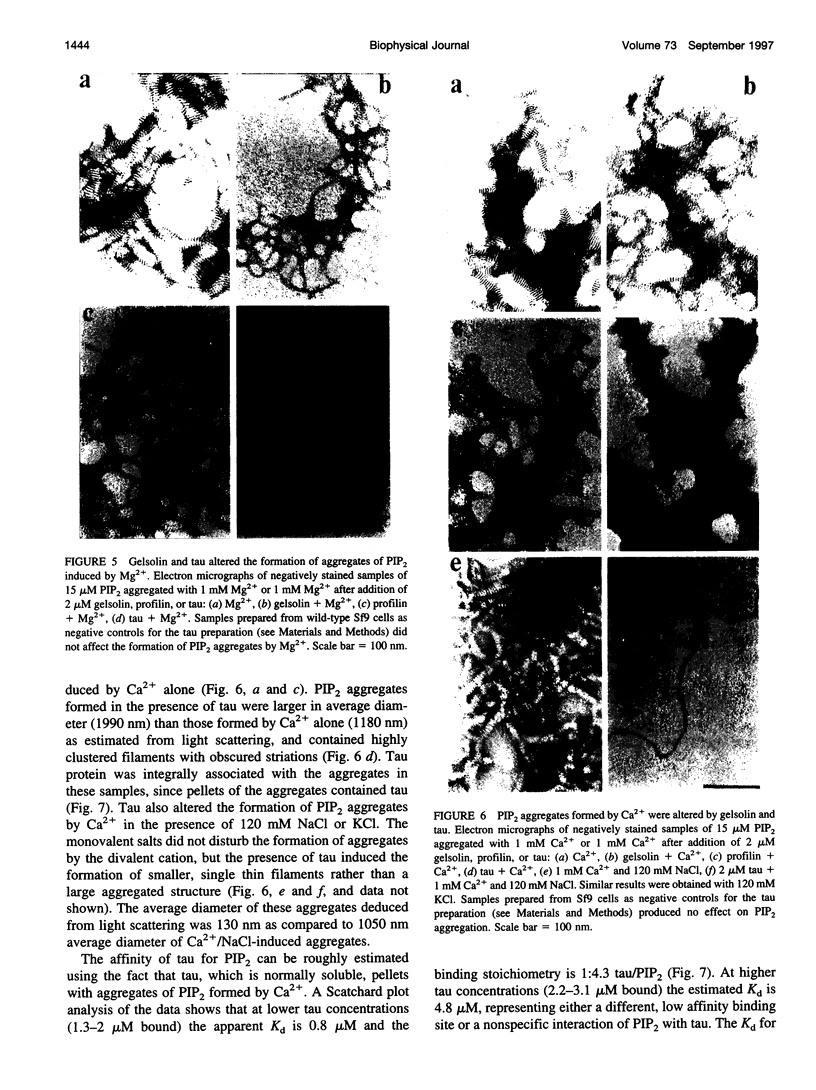
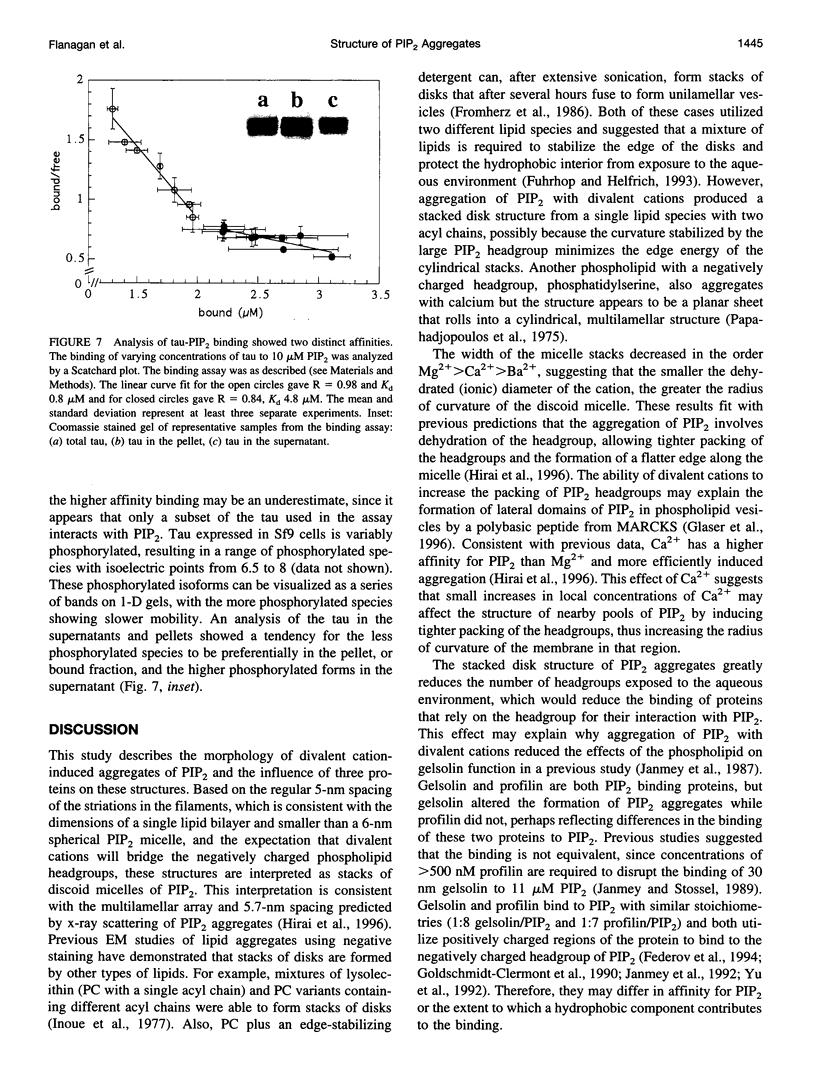


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt R., Léger J., Lee G. Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J Cell Biol. 1995 Dec;131(5):1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Jian, Profit Adam A., Prestwich Glenn D. Synthesis of Photoactivatable 1,2-O-Diacyl-sn-glycerol Derivatives of 1-L-Phosphatidyl-D-myo-inositol 4,5-Bisphosphate (PtdInsP(2)) and 3,4,5-Trisphosphate (PtdInsP(3)). J Org Chem. 1996 Sep 6;61(18):6305–6312. doi: 10.1021/jo960895r. [DOI] [PubMed] [Google Scholar]
- Divecha N., Banfić H., Irvine R. F. Inositides and the nucleus and inositides in the nucleus. Cell. 1993 Aug 13;74(3):405–407. doi: 10.1016/0092-8674(93)80041-c. [DOI] [PubMed] [Google Scholar]
- Divecha N., Irvine R. F. Phospholipid signaling. Cell. 1995 Jan 27;80(2):269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Fedorov A. A., Magnus K. A., Graupe M. H., Lattman E. E., Pollard T. D., Almo S. C. X-ray structures of isoforms of the actin-binding protein profilin that differ in their affinity for phosphatidylinositol phosphates. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8636–8640. doi: 10.1073/pnas.91.18.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Busciglio J., Cáceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Brain Res Dev Brain Res. 1989 Oct 1;49(2):215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Fullington J. G., Hendrickson H. S. Phospholipid-metal complexes. Interaction of triphosphoinositide- and phosphatidylserine-metal complexes with ethylenediamine, polyaminoacids, and protein. J Biol Chem. 1966 Sep 10;241(17):4098–4100. [PubMed] [Google Scholar]
- Glaser M., Wanaski S., Buser C. A., Boguslavsky V., Rashidzada W., Morris A., Rebecchi M., Scarlata S. F., Runnels L. W., Prestwich G. D. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem. 1996 Oct 18;271(42):26187–26193. doi: 10.1074/jbc.271.42.26187. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Bokoch G. M., Carpenter C. L., Janmey P. A., Taylor L. A., Toker A., Stossel T. P. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995 Aug 25;82(4):643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Fullington J. G. Stabilities of metal complexes of phospholipids: Ca(II), Mg(II), and Ni(II) complexes of phosphatidylserine and triphosphoinositide. Biochemistry. 1965 Aug;4(8):1599–1605. doi: 10.1021/bi00884a021. [DOI] [PubMed] [Google Scholar]
- Hwang S. C., Jhon D. Y., Bae Y. S., Kim J. H., Rhee S. G. Activation of phospholipase C-gamma by the concerted action of tau proteins and arachidonic acid. J Biol Chem. 1996 Aug 2;271(31):18342–18349. doi: 10.1074/jbc.271.31.18342. [DOI] [PubMed] [Google Scholar]
- Inoue K., Suzuki K., Nojima S. Morphology of lipid micelles containing lysolecithin. J Biochem. 1977 Apr;81(4):1097–1106. doi: 10.1093/oxfordjournals.jbchem.a131534. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Iida K., Yin H. L., Stossel T. P. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem. 1987 Sep 5;262(25):12228–12236. [PubMed] [Google Scholar]
- Janmey P. A., Lamb J., Allen P. G., Matsudaira P. T. Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem. 1992 Jun 15;267(17):11818–11823. [PubMed] [Google Scholar]
- Janmey P. A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Janmey P. A. Polyproline affinity method for purification of platelet profilin and modification with pyrene-maleimide. Methods Enzymol. 1991;196:92–99. doi: 10.1016/0076-6879(91)96011-f. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Gelsolin-polyphosphoinositide interaction. Full expression of gelsolin-inhibiting function by polyphosphoinositides in vesicular form and inactivation by dilution, aggregation, or masking of the inositol head group. J Biol Chem. 1989 Mar 25;264(9):4825–4831. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Kapeller R., Cantley L. C. Phosphatidylinositol 3-kinase. Bioessays. 1994 Aug;16(8):565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- Knops J., Kosik K. S., Lee G., Pardee J. D., Cohen-Gould L., McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991 Aug;114(4):725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Practical aspects of immune electron microscopy. Methods Enzymol. 1979;61:250–257. doi: 10.1016/0076-6879(79)61014-5. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Ling L. E., Schulz J. T., Cantley L. C. Characterization and purification of membrane-associated phosphatidylinositol-4-phosphate kinase from human red blood cells. J Biol Chem. 1989 Mar 25;264(9):5080–5088. [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Rupert L. A., van Breemen J. F., van Bruggen E. F., Engberts J. B., Hoekstra D. Calcium-induced fusion of didodecylphosphate vesicles: the lamellar to hexagonal II (HII) phase transition. J Membr Biol. 1987;95(3):255–263. doi: 10.1007/BF01869487. [DOI] [PubMed] [Google Scholar]
- Sugiura Y. Structure of molecular aggregates of 1-(3-sn-phosphatidyl)-L-myo-inositol 3,4-bis(phosphate) in water. Biochim Biophys Acta. 1981 Feb 20;641(1):148–159. doi: 10.1016/0005-2736(81)90578-2. [DOI] [PubMed] [Google Scholar]
- Surridge C. D., Burns R. G. The difference in the binding of phosphatidylinositol distinguishes MAP2 from MAP2C and Tau. Biochemistry. 1994 Jul 5;33(26):8051–8057. doi: 10.1021/bi00192a009. [DOI] [PubMed] [Google Scholar]
- Thurston V. C., Zinkowski R. P., Binder L. I. Tau as a nucleolar protein in human nonneural cells in vitro and in vivo. Chromosoma. 1996 Jul;105(1):20–30. doi: 10.1007/BF02510035. [DOI] [PubMed] [Google Scholar]
- Wang Y., Loomis P. A., Zinkowski R. P., Binder L. I. A novel tau transcript in cultured human neuroblastoma cells expressing nuclear tau. J Cell Biol. 1993 Apr;121(2):257–267. doi: 10.1083/jcb.121.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. X., Sun H. Q., Janmey P. A., Yin H. L. Identification of a polyphosphoinositide-binding sequence in an actin monomer-binding domain of gelsolin. J Biol Chem. 1992 Jul 25;267(21):14616–14621. [PubMed] [Google Scholar]