Abstract
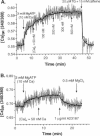
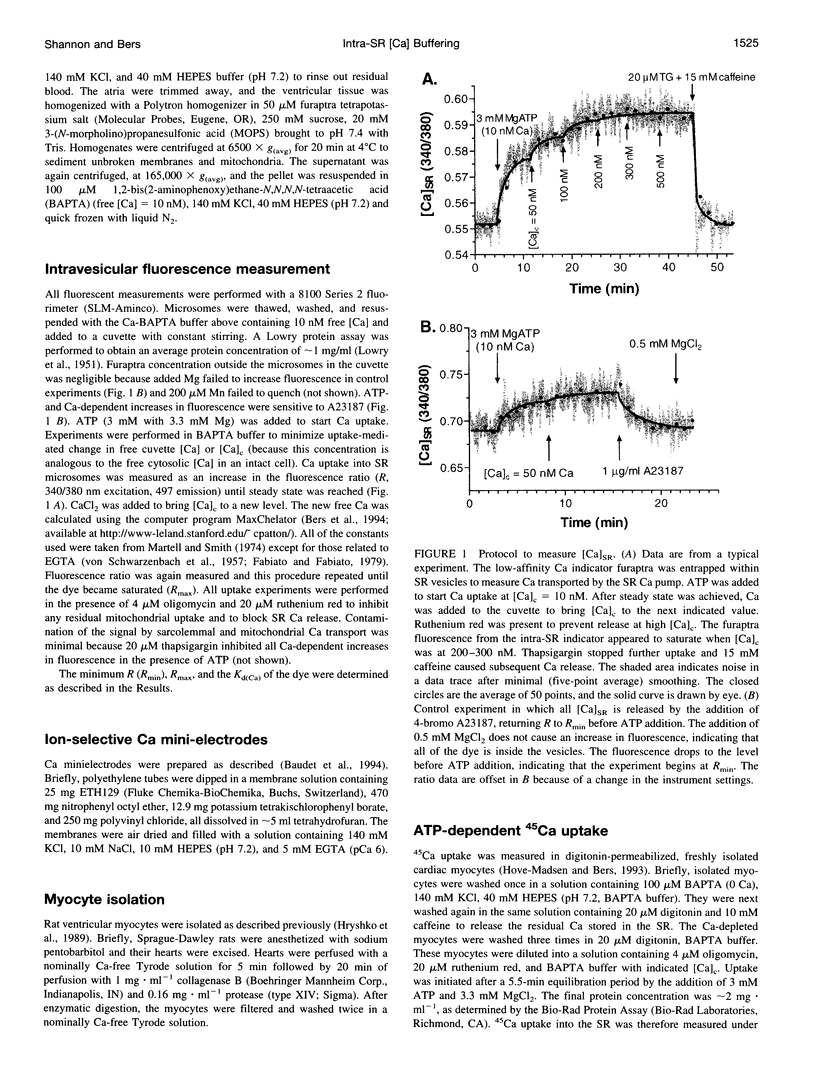
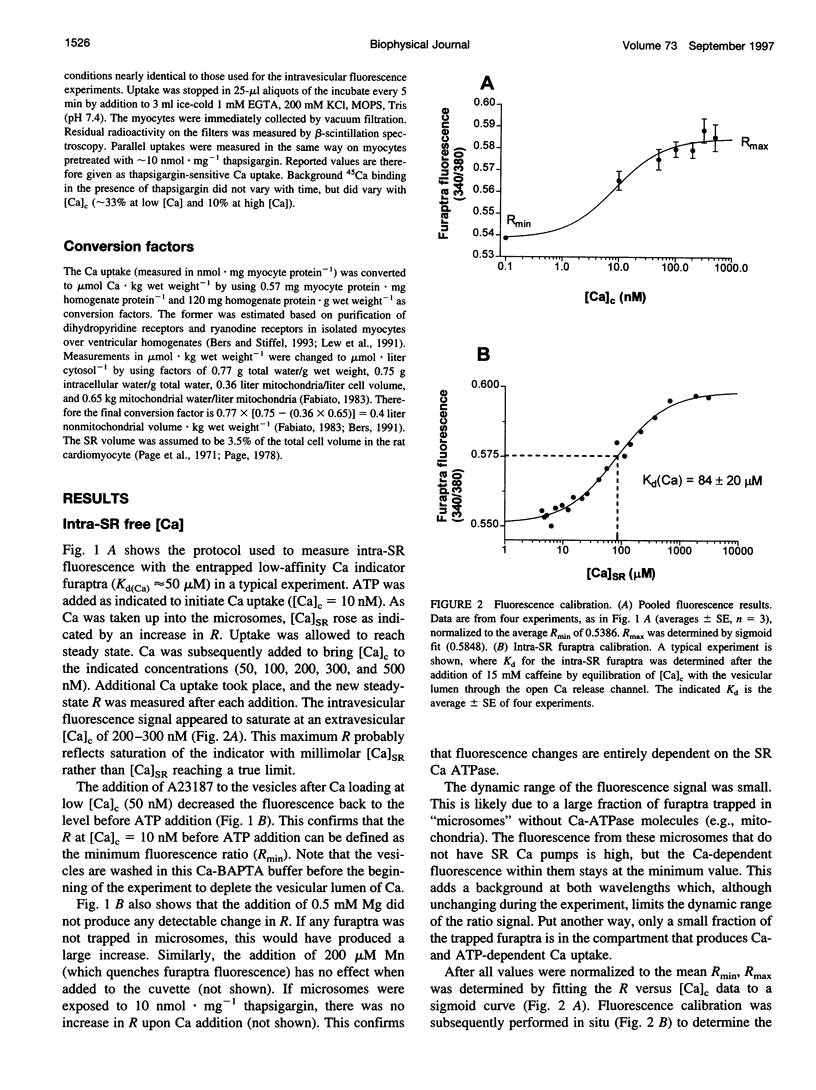
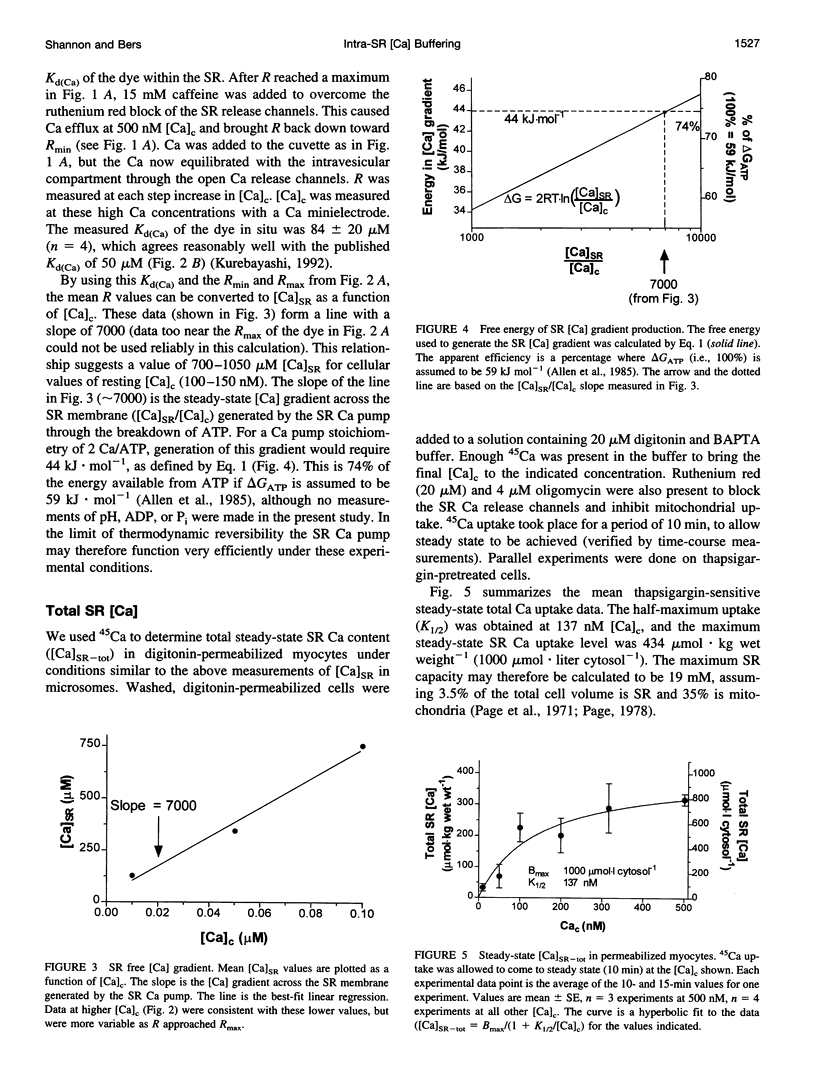
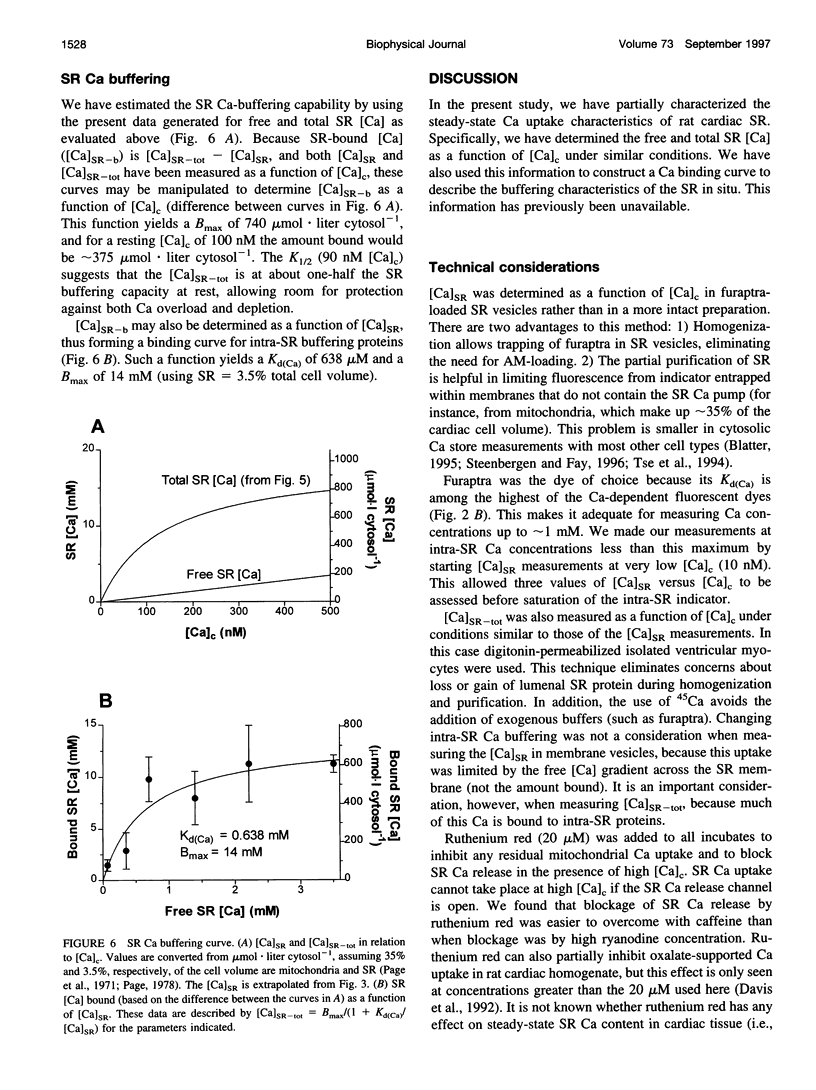
To measure the free intrasarcoplasmic reticulum [Ca] ([Ca]SR) in isolated rat cardiac microsomes, ventricular tissue was homogenized in the presence of the low-affinity Ca indicator furaptra. Stepwise increases in cuvette [Ca] ([Ca]c) in the presence of ATP caused progressive increases in steady-state intravesicular fluorescence ratio to a maximum (Rmax). Steady-state [Ca]SR/[Ca]c was approximately 7000. Therefore the resting [Ca]SR may approach 700 microM in the rat cardiac myocyte at [Ca]c = 100 nM. The sarcoplasmic reticulum (SR) Ca pump requires a free energy of deltaG approximately 44 kJ x mol(-1) to generate this [Ca] gradient (e.g., approximately 74% of deltaG(ATP)). Total SR 45Ca uptake was also measured in digitonin-permeabilized myocytes as a function of [Ca]c in the absence of precipitating ions. The steady-state SR Ca content at 100 nM [Ca]c was approximately 400 micromol/liter cytosolic volume. Used together, these data allowed evaluation of the in situ SR Ca-buffering properties. The SR Ca-binding site concentration was approximately 14 mM, and Kd(Ca) approximately 0.638 mM [Ca]SR.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994 Apr 15;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani R. A., Bassani J. W., Bers D. M. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca ATPase. Pflugers Arch. 1995 Aug;430(4):573–578. doi: 10.1007/BF00373894. [DOI] [PubMed] [Google Scholar]
- Bassani R. A., Bers D. M. Rate of diastolic Ca release from the sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys J. 1995 May;68(5):2015–2022. doi: 10.1016/S0006-3495(95)80378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet S., Hove-Madsen L., Bers D. M. How to make and use calcium-specific mini- and microelectrodes. Methods Cell Biol. 1994;40:93–113. [PubMed] [Google Scholar]
- Beeler T. J. Ca2+ uptake and membrane potential in sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Oct 10;255(19):9156–9161. [PubMed] [Google Scholar]
- Bers D. M., Stiffel V. M. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol. 1993 Jun;264(6 Pt 1):C1587–C1593. doi: 10.1152/ajpcell.1993.264.6.C1587. [DOI] [PubMed] [Google Scholar]
- Blatter L. A. Depletion and filling of intracellular calcium stores in vascular smooth muscle. Am J Physiol. 1995 Feb;268(2 Pt 1):C503–C512. doi: 10.1152/ajpcell.1995.268.2.C503. [DOI] [PubMed] [Google Scholar]
- Bridge J. H. Relationships between the sarcoplasmic reticulum and sarcolemmal calcium transport revealed by rapidly cooling rabbit ventricular muscle. J Gen Physiol. 1986 Oct;88(4):437–473. doi: 10.1085/jgp.88.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol. 1989 Jul;257(1 Pt 1):C147–C152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H., Jorgensen A. O., Mintzer M. C. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem. 1983 Jan 25;258(2):1197–1204. [PubMed] [Google Scholar]
- Chen W., Steenbergen C., Levy L. A., Vance J., London R. E., Murphy E. Measurement of free Ca2+ in sarcoplasmic reticulum in perfused rabbit heart loaded with 1,2-bis(2-amino-5,6-difluorophenoxy)ethane-N,N,N',N'-tetraacetic acid by 19F NMR. J Biol Chem. 1996 Mar 29;271(13):7398–7403. [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Dani A. M., Cittadini A., Inesi G. Calcium transport and contractile activity in dissociated mammalian heart cells. Am J Physiol. 1979 Sep;237(3):C147–C155. doi: 10.1152/ajpcell.1979.237.3.C147. [DOI] [PubMed] [Google Scholar]
- Davis M. D., Lebolt W., Feher J. J. Reversibility of the effects of normothermic global ischemia on the ryanodine-sensitive and ryanodine-insensitive calcium uptake of cardiac sarcoplasmic reticulum. Circ Res. 1992 Jan;70(1):163–171. doi: 10.1161/01.res.70.1.163. [DOI] [PubMed] [Google Scholar]
- Delbridge L. M., Bassani J. W., Bers D. M. Steady-state twitch Ca2+ fluxes and cytosolic Ca2+ buffering in rabbit ventricular myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C192–C199. doi: 10.1152/ajpcell.1996.270.1.C192. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Hove-Madsen L., Bers D. M. Passive Ca buffering and SR Ca uptake in permeabilized rabbit ventricular myocytes. Am J Physiol. 1993 Mar;264(3 Pt 1):C677–C686. doi: 10.1152/ajpcell.1993.264.3.C677. [DOI] [PubMed] [Google Scholar]
- Hryshko L. V., Stiffel V., Bers D. M. Rapid cooling contractures as an index of sarcoplasmic reticulum calcium content in rabbit ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1369–H1377. doi: 10.1152/ajpheart.1989.257.5.H1369. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Berkoff H. A. Measurement of rapidly exchangeable cellular calcium in the perfused beating rat heart. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5665–5668. doi: 10.1073/pnas.78.9.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P., Inui M., Tada M., Chiesi M., Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989 Nov 2;342(6245):90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Kawai M., Konishi M. Measurement of sarcoplasmic reticulum calcium content in skinned mammalian cardiac muscle. Cell Calcium. 1994 Aug;16(2):123–136. doi: 10.1016/0143-4160(94)90007-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitsky D. O., Benevolensky D. S., Levchenko T. S., Smirnov V. N., Chazov E. I. Calcium-binding rate and capacity of cardiac sarcoplasmic reticulum. J Mol Cell Cardiol. 1981 Sep;13(9):785–796. doi: 10.1016/0022-2828(81)90236-4. [DOI] [PubMed] [Google Scholar]
- Levitsky D., de la Bastie D., Schwartz K., Lompré A. M. Ca(2+)-ATPase and function of sarcoplasmic reticulum during cardiac hypertrophy. Am J Physiol. 1991 Oct;261(4 Suppl):23–26. doi: 10.1152/ajplung.1991.261.4.L23. [DOI] [PubMed] [Google Scholar]
- Lew W. Y., Hryshko L. V., Bers D. M. Dihydropyridine receptors are primarily functional L-type calcium channels in rabbit ventricular myocytes. Circ Res. 1991 Oct;69(4):1139–1145. doi: 10.1161/01.res.69.4.1139. [DOI] [PubMed] [Google Scholar]
- Meissner G. Calcium transport and monovalent cation and proton fluxes in sarcoplasmic reticulum vesicles. J Biol Chem. 1981 Jan 25;256(2):636–643. [PubMed] [Google Scholar]
- Mitchell R. D., Simmerman H. K., Jones L. R. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988 Jan 25;263(3):1376–1381. [PubMed] [Google Scholar]
- Page E., McCallister L. P., Power B. Sterological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1465–1466. doi: 10.1073/pnas.68.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Scott B. T., Simmerman H. K., Collins J. H., Nadal-Ginard B., Jones L. R. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988 Jun 25;263(18):8958–8964. [PubMed] [Google Scholar]
- Slupsky J. R., Ohnishi M., Carpenter M. R., Reithmeier R. A. Characterization of cardiac calsequestrin. Biochemistry. 1987 Oct 6;26(20):6539–6544. doi: 10.1021/bi00394a038. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Briggs F. N. Estimating the functional capabilities of sarcoplasmic reticulum in cardiac muscle. Calcium binding. Circ Res. 1974 Apr;34(4):531–540. doi: 10.1161/01.res.34.4.531. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature. 1977 Aug 11;268(5620):556–558. doi: 10.1038/268556a0. [DOI] [PubMed] [Google Scholar]
- Steenbergen J. M., Fay F. S. The quantal nature of calcium release to caffeine in single smooth muscle cells results from activation of the sarcoplasmic reticulum Ca(2+)-ATPase. J Biol Chem. 1996 Jan 26;271(4):1821–1824. doi: 10.1074/jbc.271.4.1821. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Wang J. H. Stimulation of bovine cardiac sarcoplasmic reticulum Ca2+ pump and blocking of phospholamban phosphorylation and dephosphorylation by a phospholamban monoclonal antibody. J Biol Chem. 1986 May 25;261(15):7018–7023. [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Katz A. M. Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Apr 10;250(7):2640–2647. [PubMed] [Google Scholar]
- Terracciano C. M., Naqvi R. U., MacLeod K. T. Effects of rest interval on the release of calcium from the sarcoplasmic reticulum in isolated guinea pig ventricular myocytes. Circ Res. 1995 Aug;77(2):354–360. doi: 10.1161/01.res.77.2.354. [DOI] [PubMed] [Google Scholar]
- Tse F. W., Tse A., Hille B. Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+ oscillations. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9750–9754. doi: 10.1073/pnas.91.21.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A., Negretti N., Hester S. B., Eisner D. A. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch. 1993 Apr;423(1-2):158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- de la Bastie D., Levitsky D., Rappaport L., Mercadier J. J., Marotte F., Wisnewsky C., Brovkovich V., Schwartz K., Lompré A. M. Function of the sarcoplasmic reticulum and expression of its Ca2(+)-ATPase gene in pressure overload-induced cardiac hypertrophy in the rat. Circ Res. 1990 Feb;66(2):554–564. doi: 10.1161/01.res.66.2.554. [DOI] [PubMed] [Google Scholar]