Abstract
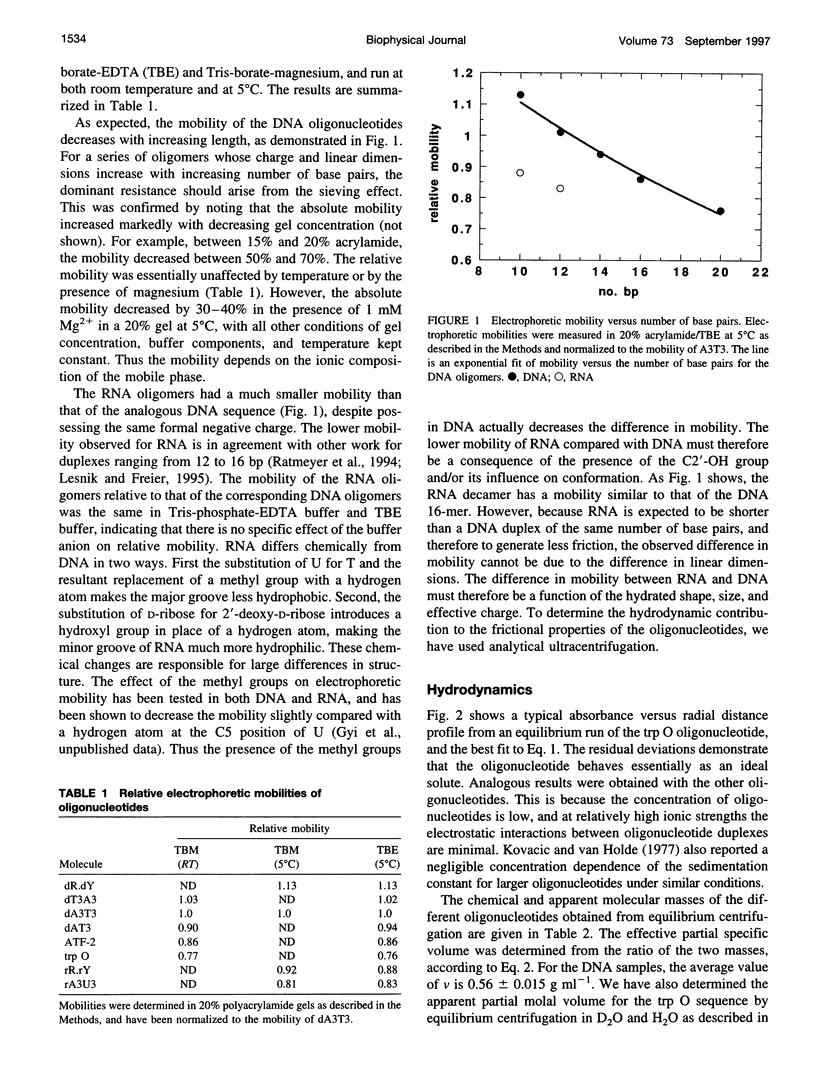
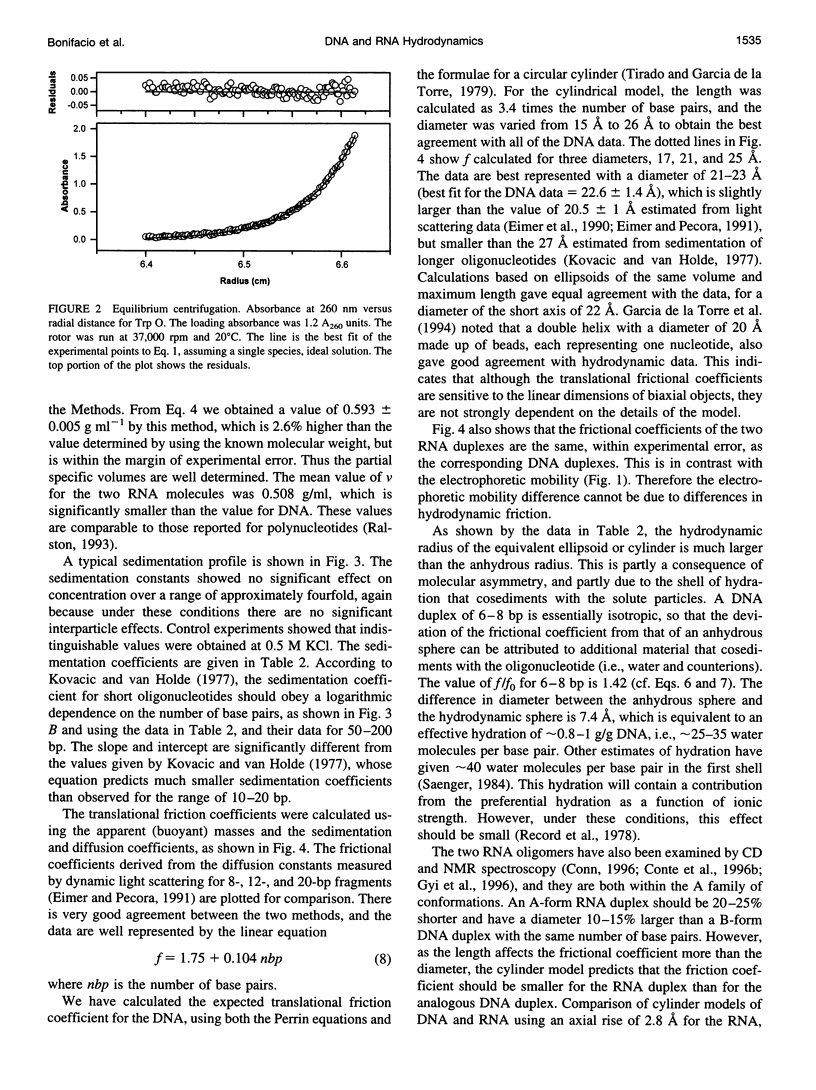
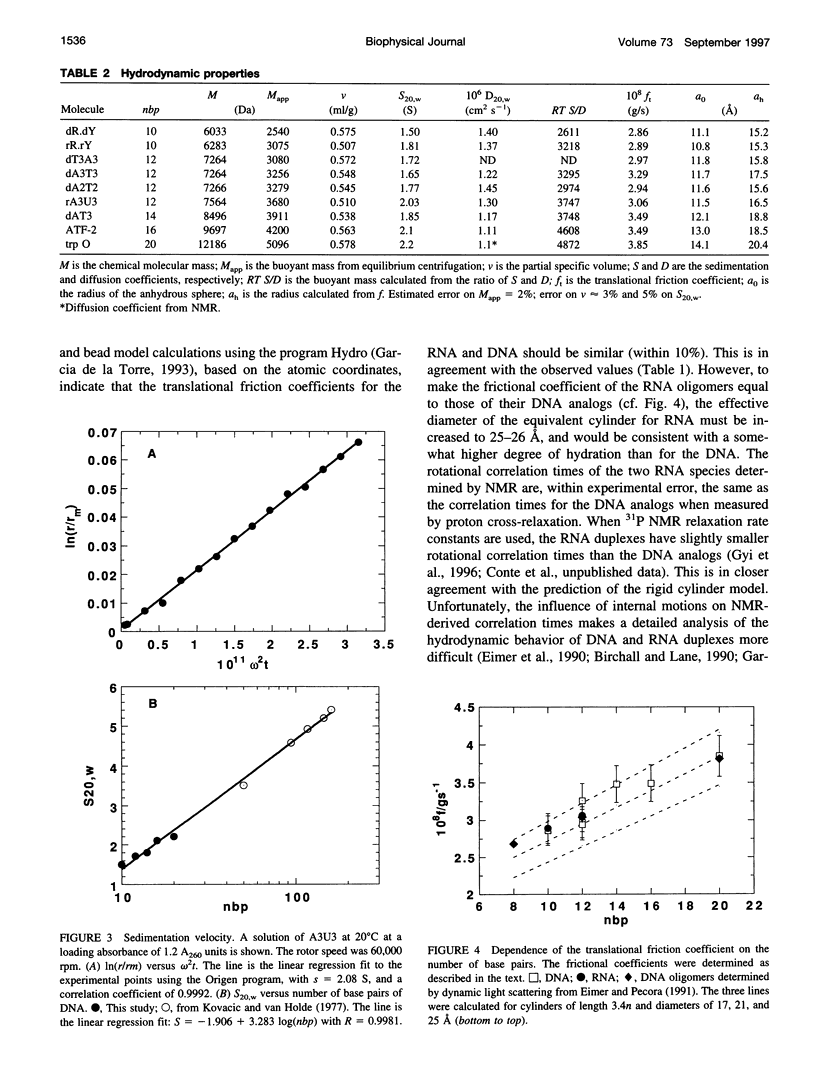
The electrophoretic behavior of defined DNA and RNA oligonucleotide duplexes from 10 to 20 bp in length has been investigated as a function of salt conditions, gel concentration, and temperature. The RNA oligomers migrated much more slowly than the DNA oligomers of the same sequence under all conditions. From sedimentation equilibrium and velocity measurements, the apparent partial specific volume in 0.1 M KCI, 20 mM NaPi, pH 7, was determined as 0.56 +/- 0.015 ml g(-1) for DNA and 0.508 ml g(-1) for RNA. The translational friction coefficients were determined and compared with the values calculated for cylinders. Taking into account the shape factors, the solution density, and partial specific volumes, the effective degree of hydration was estimated as 0.8-1 g g(-1) DNA. There was no significant difference in the frictional coefficients of the DNA and RNA oligomers, indicating that the effective sizes of DNA and RNA are very similar in solution. The differential electrophoretic mobility of DNA and RNA must arise from the differences in interaction with counterions, which is probably a global property of the oligonucleotides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birchall A. J., Lane A. N. Anisotropic rotation in nucleic acid fragments: significance for determination of structures from NMR data. Eur Biophys J. 1990;19(2):73–78. doi: 10.1007/BF00185089. [DOI] [PubMed] [Google Scholar]
- Brown D. G., Sanderson M. R., Skelly J. V., Jenkins T. C., Brown T., Garman E., Stuart D. I., Neidle S. Crystal structure of a berenil-dodecanucleotide complex: the role of water in sequence-specific ligand binding. EMBO J. 1990 Apr;9(4):1329–1334. doi: 10.1002/j.1460-2075.1990.tb08242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Brown D. J. Purification of synthetic DNA. Methods Enzymol. 1992;211:20–35. doi: 10.1016/0076-6879(92)11004-3. [DOI] [PubMed] [Google Scholar]
- Chalikian T. V., Sarvazyan A. P., Plum G. E., Breslauer K. J. Influence of base composition, base sequence, and duplex structure on DNA hydration: apparent molar volumes and apparent molar adiabatic compressibilities of synthetic and natural DNA duplexes at 25 degrees C. Biochemistry. 1994 Mar 8;33(9):2394–2401. doi: 10.1021/bi00175a007. [DOI] [PubMed] [Google Scholar]
- Conte M. R., Bauer C. J., Lane A. N. Determination of sugar conformations by NMR in larger DNA duplexes using both dipolar and scalar data: application to d(CATGTGACGTCACATG)2. J Biomol NMR. 1996 May;7(3):190–206. doi: 10.1007/BF00202036. [DOI] [PubMed] [Google Scholar]
- Conte M. R., Conn G. L., Brown T., Lane A. N. Hydration of the RNA duplex r(CGCAAAUUUGCG)2 determined by NMR. Nucleic Acids Res. 1996 Oct 1;24(19):3693–3699. doi: 10.1093/nar/24.19.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M., Portmann S., Usman N. RNA hydration: a detailed look. Biochemistry. 1996 Jul 2;35(26):8489–8494. doi: 10.1021/bi9607214. [DOI] [PubMed] [Google Scholar]
- Eimer W., Williamson J. R., Boxer S. G., Pecora R. Characterization of the overall and internal dynamics of short oligonucleotides by depolarized dynamic light scattering and NMR relaxation measurements. Biochemistry. 1990 Jan 23;29(3):799–811. doi: 10.1021/bi00455a030. [DOI] [PubMed] [Google Scholar]
- Garcia de la Torre J., Navarro S., Lopez Martinez M. C. Hydrodynamic properties of a double-helical model for DNA. Biophys J. 1994 May;66(5):1573–1579. doi: 10.1016/S0006-3495(94)80949-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyi J. I., Conn G. L., Lane A. N., Brown T. Comparison of the thermodynamic stabilities and solution conformations of DNA.RNA hybrids containing purine-rich and pyrimidine-rich strands with DNA and RNA duplexes. Biochemistry. 1996 Sep 24;35(38):12538–12548. doi: 10.1021/bi960948z. [DOI] [PubMed] [Google Scholar]
- Jenkins T. C., Lane A. N., Neidle S., Brown D. G. NMR and molecular modeling studies of the interaction of berenil and pentamidine with d(CGCAAATTTGCG)2. Eur J Biochem. 1993 May 1;213(3):1175–1184. doi: 10.1111/j.1432-1033.1993.tb17868.x. [DOI] [PubMed] [Google Scholar]
- Kovacic R. T., van Holde K. E. Sedimentation of homogeneous double-strand DNA molecules. Biochemistry. 1977 Apr 5;16(7):1490–1498. doi: 10.1021/bi00626a038. [DOI] [PubMed] [Google Scholar]
- Lane A. N., Jenkins T. C., Frenkiel T. A. Hydration and solution structure of d(CGCAAATTTGCG)2 and its complex with propamidine from NMR and molecular modelling. Biochim Biophys Acta. 1997 Feb 7;1350(2):205–220. doi: 10.1016/s0167-4781(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Lane A. N. The solution conformations of a mutant trp operator determined by n.m.r. spectroscopy. Biochem J. 1991 Jan 15;273(Pt 2):383–391. doi: 10.1042/bj2730383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre J. F., Lane A. N., Jardetzky O. Solution structure of the Trp operator of Escherichia coli determined by NMR. Biochemistry. 1987 Aug 11;26(16):5076–5090. doi: 10.1021/bi00390a029. [DOI] [PubMed] [Google Scholar]
- Lesnik E. A., Freier S. M. Relative thermodynamic stability of DNA, RNA, and DNA:RNA hybrid duplexes: relationship with base composition and structure. Biochemistry. 1995 Aug 29;34(34):10807–10815. doi: 10.1021/bi00034a013. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Mills P. A., Rashid A., James T. L. Monte Carlo calculations of ion distributions surrounding the oligonucleotide d(ATATATATAT)2 in the B, A, and wrinkled D conformations. Biopolymers. 1992 Nov;32(11):1491–1501. doi: 10.1002/bip.360321108. [DOI] [PubMed] [Google Scholar]
- Otting G., Liepinsh E., Wüthrich K. Protein hydration in aqueous solution. Science. 1991 Nov 15;254(5034):974–980. doi: 10.1126/science.1948083. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Ratmeyer L., Vinayak R., Zhong Y. Y., Zon G., Wilson W. D. Sequence specific thermodynamic and structural properties for DNA.RNA duplexes. Biochemistry. 1994 May 3;33(17):5298–5304. doi: 10.1021/bi00183a037. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Wahl M. C., Ban C., Sekharudu C., Ramakrishnan B., Sundaralingam M. Structure of the purine-pyrimidine alternating RNA double helix, r(GUAUAUA)d(C), with a 3'-terminal deoxy residue. Acta Crystallogr D Biol Crystallogr. 1996 Jul 1;52(Pt 4):655–667. doi: 10.1107/S0907444996000248. [DOI] [PubMed] [Google Scholar]


