Abstract
Lung inflammation, infection and injury can lead to critical illness and death. The current means to pharmacologically treat excessive uncontrolled lung inflammation needs improvement because many treatments are or will become immunosuppressive. The inflammatory response evolved to protect the host from microbes, injury and environmental insults. This response brings phagocytes from the bloodstream to the tissue site to phagocytize and neutralize bacterial invaders and enables airway anti-microbial functions. This physiologic response is ideally self-limited with initiation and resolution phases. Polyunsaturated essential fatty acids (PUFA) are precursors to potent molecules that govern both phases. In the initiation phase, arachidonic acid is converted to prostaglandins and leukotrienes that activate leukocytes to transmigrate from post-capillary venules. The omega-3 fatty acids (e.g. DHA and EPA) are precursors to resolvins, protectins and maresins, which are families of chemically distinct mediators with potent functions in resolution of acute and chronic inflammation in the respiratory system.
Keywords: efferocytosis, proresolving mediators, polyunsaturated fatty acid, lung disease, cysteinyl-specialized proresolving mediators, resolvins
Introduction
The respiratory system has the vital function of delivering oxygen to the circulating blood and hence is highly vascularized. The respiratory system from the nose, sinus, and upper and lower airways to the distal lung is protected from invaders by the innate and adaptive immune system. Resident immune cells of the lung and phagocytes delivered by the pulmonary circulation protect and defend the respiratory system from infection, injury and excess inflammation. The essential polyunsaturated fatty acids (PUFAs), arachidonic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are precursors to many chemical signals that instruct cell responses and functions in the respiratory system. For example, arachidonic acid is precursor to eicosanoids including prostaglandins, thromboxanes, leukotrienes and lipoxins that play critical roles in the pulmonary circulation, coagulation and inflammation. Platelet-derived thromboxane and vascular endothelial cell prostacyclin are key lipid mediators in vascular tone and coagulation (1). Leukotrienes such as leukotriene B4 (LTB4), the potent chemoattractant, are critical in host defense and bring circulating phagocytes to the site of injury or microbial invasion in the respiratory system. The slow-reacting substance of anaphylaxis (SRS-A), or leukotrienes (LT) LTC4, LTD4 and LTE4, are potent smooth muscle contractors in the respiratory system produced by mast cells, eosinophils, and macrophages, including alveolar macrophages (2). The structures and mechanisms of the enzymes involved in the biosynthesis of the leukotrienes are fully elucidated, as reviewed recently in Haeggström and Newcomer (3). There is increasing evidence from large-scale randomized human clinical trials that the omega-3 essential PUFA EPA and DHA play critical roles in human heart and lung health (4-10). The acute inflammatory response is divided on the basis of cellular infiltrates by pathologists (11) into initiation and resolution phases. The arachidonic acid-derived eicosanoids prostaglandins and leukotrienes help mount leukocyte tissue infiltration, and the omega-3 PUFA DHA and EPA are precursors to resolvins, protectins and maresins, which are predominantly biosynthesized temporally during the resolution phase and function to promote resolution of inflammatory exudates and tissue infiltrates (12-14). These specialized proresolving molecules (SPM) and their biosynthetic pathways were the subject of earlier Annual Reviews. Interested readers are directed to these extensive reviews of the foundational literature (15-17) for detailed accounts. The role of nutrition in resolving inflammation was also the subject of an earlier review by Zhang and Spite (18). The resolvins and SPMs, given their potent anti-inflammatory and proresolving function, open the terrain of resolution biology and pharmacology as new approaches to control excessive inflammation to many investigations, since these novel molecules are now commercially available. Uncontrolled inflammation is today associated with many widely occurring chronic human diseases, heightening the interest in new approaches to controlling the deleterious outcomes of these diseases via exploring agonists of endogenous resolution mechanisms, since the current approaches have some unwanted side effects, including immunosuppression (19).
Authoritative recent reviews on new approaches with resolution pharmacology provide a solid analysis of this new field for readers (see (20, 21) in the Annual Review series). Ji also reviewed initial results for the Annual Review of Pharmacology and Toxicology demonstrating the potent actions of SPM in reducing pain and itch in animal models (22). It has been 10 years since our first review of SPM in the resolution of acute lung inflammation (17). In this Annual Review, we present and review the major steps taken and advances in our appreciation of the function of SPM and novel, more recently uncovered cys-SPM in the control of resolution of inflammation in the respiratory system since our earlier annual review. The therapeutic potential of resolvins in pulmonary diseases (23) and their major structure-function relationships have recently been recognized by others and rigorously reviewed (24), which further underscores our original interest in comprehensively studying the novel molecules produced de novo in the resolution phase of the acute inflammatory response to guide us to new approaches to control excessive and chronic inflammation and collateral tissue damage in the respiratory system relevant in lung injury, sepsis and other life-threatening scenarios that today open new concepts in resolution medicine.
Since our earlier AR review together in 2014 focusing on resolution of inflammation in lung (17) and in our more general resolution of inflammation review (19) highlighting the emergence of the super-family of pro-resolving mediators and fundamental cellular mechanisms in the biosynthesis and functions of pro-resolving mediators, there are many exciting new developments in this period since the 2018 review , in this very rapidly growing field of pro-resolving lipid mediators in general and in the respiratory system. Here, a few of the very impactful discoveries from investigators around the world are briefly highlighted:
Human in vivo production of the resolvins, protectins and maresins (SPM) is independently confirmed and well documented by others (25, 26) including in human adolescents (27) and in young adults in a population study of 978 subjects at 27 years old (28).
Resolvin E1, which displays function in the lung and in cardiovascular disease models, is diminished in humans with adiposity (28), elegantly demonstrated using LC-MS-MS targeted metabololipidomics.
Human SPM tissue amounts are dependent on consumption of the n-3 essential fatty acids EPA and DHA precursors (29) with SPMs dysregulated in SARS-COVID infections in humans (30).
In human airway, SPM are identified in chronic rhinosinusitis patients (31) and discovered to play an important role in cystic fibrosis (32, 33) as a potential marker of disease (34) and therapeutic potential (35).
Human Vagus nerve on electrical stimulation ex vivo releases SPMs and reduces prostaglandins (36).
SPM are regulated by low-dose carbon-monoxide in non-human primates (37).
Complete stereochemistry and total organic synthesis of the cysteinyl-containing maresins, protectins and resolvins are established (38) and commercially available.
cys-Maresins counter-regulate the actions of cys-leukotrienes (39) relevant to human asthma (40).
The resolvin D2 receptor was uncovered (41), and agonist antibody to the resolvin E1 receptor was introduced and demonstrated to be a potent stimulant of endogenous resolution programs as a promising new therapeutic immunoresolvent for chronic inflammatory diseases (42) and are now in clinical development.
A role for SPMs in the pathogenesis of organ fibrosis and pulmonary fibrosis is recognized (43).
These are just some of the exciting advances in this rapidly growing new field of SPMs in resolution medicine that can impact airway diseases, cardiovascular and many other diseases characterized by excessive inflammation (44).
Resolvin Biosynthesis and Role of Activated Vascular Endothelium
The first resolvin, termed Resolvin E1, was isolated from inflammatory exudates produced in vivo in self-limited acute inflammatory response in the resolution phase (12) and proved to be a potent bioactive molecule produced from EPA. The structure of Resolvin E1 was presented at the International Eicosanoid meeting that year (2000) in Florence, Italy. Resolvin E1 structure was elucidated, and it was shown to stop leukocyte transendothelial migration and leukocyte infiltration in vivo in mice. To explore the biosynthesis with human cells, hypoxic microvascular endothelial cells were coincubated with isolated human neutrophils. The endothelial cells were activated with TNF-α and IL-1β as well as the hypoxic environment that upregulated COX-2 to mimic in vivo conditions during acute inflammation. The activated endothelial cells release omega-3 EPA that is converted by acetylated COX-2 to 18-HEPE, which can be further transformed via transcellular biosynthesis by human neutrophils to Resolvin E1 (12) in this coincubation setting designed to mimic the exudate conditions in which this novel 5,12,18-trihydroxyeicosapentaenoic acid was originally isolated as a bioactive molecule (12). The complete stereochemistry of Resolvin E1 was established by total organic synthesis and matching to the endogenous exudate-derived bioactive molecule, confirming its potent actions and proposed structure (45). Resolvin E2 was identified soon afterwards (46) as the dihydroxy bioactive product of this EPA pathway.
Activated human neutrophils in a hypoxic environment readily convert 18-HEPE to Resolvin E2 in a hypoxia chamber. The reaction mechanism to produce 18R-HEPE and 18S-HEPE with aspirin-acetylated COX-2 was next elucidated (47) and recently confirmed by Cebrián-Prats et al. (48). The biosynthesis of the E-series Resolvins was reviewed in detail recently (49), with focus on the complete stereochemistry and enzymes involved for each step of the biosynthesis. The role of 5-lipoxygenase (5-LOX) and LTA4 hydrolase in Resolvin E2 was established (46, 50) using isolated and recombinant enzymes. E-series Resolvins are locally biosynthesized, act as autacoids and are locally inactivated. Stable analogs of Resolvin E1 were prepared that demonstrate this principle (49, 51).
Resolvin E4 was uncovered more recently (52); it displays key resolution functions, i.e., limiting neutrophil infiltration and increasing macrophage efferocytosis, but also stimulates the clearance of senescent red blood cells, a physiologic function of splenic macrophages (53). The structure and function of Resolvin E4 was confirmed in two independent total organic syntheses (53, 54). E-series and D-series resolvins require vascular endothelial cells to convert EPA and DHA, respectively, to resolvin precursors to produce these potent molecules via transcellular biosynthesis (55). Both 18-HEPE and 17R-HDHA, in addition to their role as precursor, also possess potent actions of their own (56, 57). The D-series resolvins require transcellular biosynthesis, which also places them in the location where they can effectively regulate leukocyte-endothelial cell interactions and circulate to act on other cell types to regulate detachment (55, 58). Of interest, M2-like macrophages, reparative cells, can produce all of the SPMs on their own because they possess the enzymatic machinery needed to produce and release SPMs (59), including Protectins and Maresins (Figure 1 A&B) (60-62).
Figure 1A. Lipid-derived mediators in programmed resolution of the acute inflammatory response.
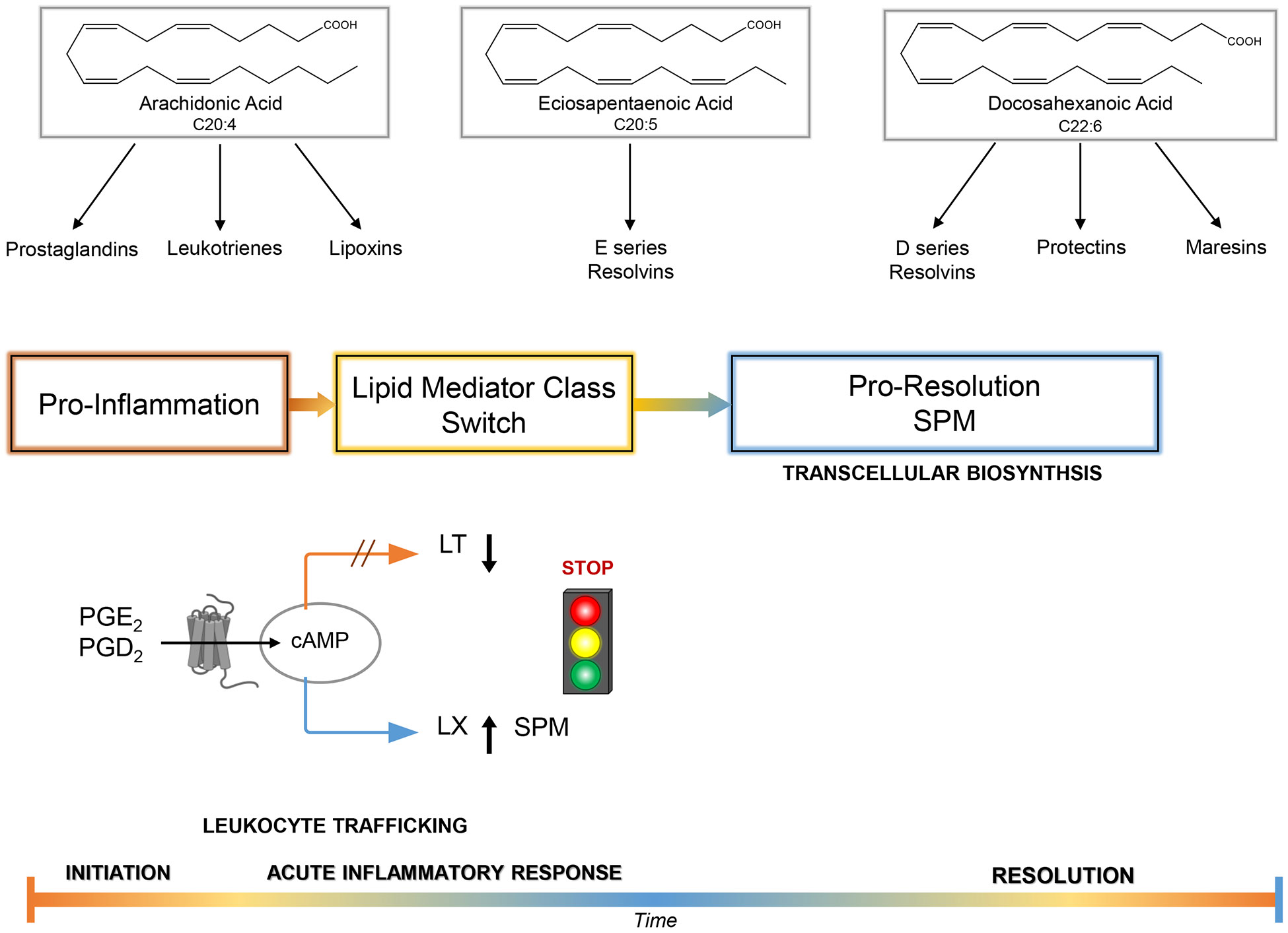
Arachidonic acid is the precursor to eicosanoids that have distinct roles as proinflammatory mediators. Prostaglandins and leukotrienes each play specific actions pivotal to the progression of inflammation. Arachidonic acid-derived epoxyeicosatetraenoic acids (EETs) produced via P450 (2, 149) and ω-3 PUFA P450 epoxides may also play roles (150, 151). Cell-cell interactions, exemplified by platelets-leukocytes transcellular biosynthesis of lipid mediators within blood vessels and/or PMN-airway epithelial cell interactions, enhance generation of lipoxins that serve as endogenous anti-inflammatory mediators self-limiting the course of inflammation (13). The essential omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid (C20:5 and C22:6) are converted to two novel families of lipid mediators, resolvins and protectins, that play pivotal roles in promoting resolution by regulating leukocyte traffic and functions. Resolvins of the E series are generated from eicosapentaenoic acid (e.g. RvE1), and resolvins of the D series (e.g., resolvin D1) and the protectins, such as neuroprotectin D1, are biosynthesized from DHA. Aspirin-triggering epimers of lipid mediators: Aspirin impacts the formation of lipoxins and resolvins by acetylating. COX-2 (e.g., in human vascular endothelial cells that stereoselectively can generate, in the case of RvE1 biosynthesis, 18R-HPEPE, which is picked up via transcellular cell-cell interactions by leukocytes and converted in a lipoxygenase-like mechanism to RvE1). The complete stereochemistry of RvE1 and at least one of its receptors were established (45). The biosynthesis of RvE1 can also be initiated by P450-like enzymes in microbes (12). Aspirin also impacts the biosynthesis of D-series resolvins. Aspirin catalytically switches COX-2 to a 17R-lipoxygenase-like mechanism that generates 17R-containing series of resolvin D and protectins (e.g., neuroprotectin D1/protectin D1; see text). Please see Serhan et al. (152) and Hamidzadeh et al. (153).
Figure 1B. Resolvins and the Acute inflammatory response in pulmonary disease.
Acute insult by way of injury (surgical intervention, trauma) or microbial invasion. Neutrophils (PMNs) traffic to the site of insult by rolling along the endothelium in the postcapillary venule, eventually adhering and transmigrating out of the vessel into the site of inflammation by swarming along a chemotactic gradient as with leukotriene B4 (154, 155). Processes such as neutrophil degranulation, neutrophil apoptosis and subsequent macrophage activation increase proinflammatory mediators such as complement components, chemokines, cytokines and cellular debris that in turn continue to promote inflammation and tissue damage. Multiple avenues for resolution medicine to intervene and promote the resolution of inflammation are illustrated;
The cell types shown to produce resolvins and the other SPM, which can impact the respiratory system, are listed in Table 1. Production of prostaglandins, leukotrienes and the proresolving mediators is temporally regulated and follows the trafficking of leukocytes in organs, including the lung (Figure 1 and Figure 2). This lipid mediator class switch is a critical component of the resolution of inflammation with the biosynthesis of specific mediators that are agonists for the resolution of inflammation (63). Both prostaglandin E2 and prostaglandin D2 stimulate the expression of the enzymes needed to biosynthesize resolution phase SPM via increasing cAMP in leukocytes (illustrated in Figure 1). Of interest, Dahlke et al demonstrated that FLAP antagonists block conversion of AA by 5-LOX to LTs and LX, but not the conversion of DHA to SPM, which requires the 5-LOX (64). In addition, antagonists of LTA4 hydrolase decrease conversion to LTB4 and induce a lipid mediator class switch to increase LTA4 conversion to lipoxins (65).
Table 1.
SPM-Producing Cell Types of the Lung
| Cell Type | SPM | Reference |
|---|---|---|
| Alveolar macrophages | RvD1 and LXA4 | Townsend et al. (160) |
| M2-like macrophages | Resolvins, protectins, maresins | Werz et al. (59) |
| Mast cells | RvD1 | Puzzovio et al. (161) |
| PMN-platelets | MaR1 | Abdulnour et al. (121) |
| PMN-airway epithelial cells | Lipoxins, RvD1 | Cox et al. (162) Isopi et al. (32) |
| Apoptotic PMN | RvDs, MaR1, LX | Dalli and Serhan (68) |
| Human monocytes Toll-like receptor 7 agonist (COVID-19 patients) | PD1, RvD5 | Koltsida et al. (163) Navarini et al. (164) |
| Eosinophils | LXA4, PDx, RvD2 | Miyata et al. (165) Serhan et al. (166) |
| Microparticles | 17-HDHA, 14-HDHA | Dalli and Serhan (68) |
DHA, docosahexaenoic acid
LX, lipoxin
Ma, maresin
PD1, protectin D1
Rv, resolvin
SPM, specialized pro-resolving mediators
Figure 2. Cell Surface Receptors for Resolvins, Protectin D1 and MaR1.

The human and mouse specialized proresolving mediator receptors display Kd/EC50 in the nM range in line with their stereoselective actions on human neutrophils, leukocytes, epithelial cells and lymphocytes. Each has been qualified with transgenic and knockout mice. Please see text for details and citations of original contributions.
Mobilization of Omega-3 Fatty Acid Substrates for SPM Production
Increasing evidence from large clinical trials in humans indicates that these essential polyunsaturated fatty acids are important to healthy lung function (7). In experimental animal models, DHA is mobilized from lymph nodes to produce Resolvin D1 and Protectin D1 via the secreted phospholipase A2 (sPLA2) group IID enzyme (66), coined the resolving PLA2. This sPLA2 also releases precursors 14-DHA and 17-HDHA from human and mouse microparticles (67, 68). In human neural tissues such as microglia, evidence for cPLA2 to release DHA for NPD1 biosynthesis is available (58, 69).
Vascular leak during inflammation produces edema that can carry DHA and EPA into inflammatory exudates, likely via albumin, to evoke exudate biosynthesis of SPMs to limit further neutrophil recruitment (70). Evidence was also recently obtained for the resolving sPLA acting on triglyceride substrate in M2-like human macrophages to liberate EPA for Resolvin E4 biosynthesis (52). The predominance of these mobilizations of substrate in the respiratory system remains a subject of continued research in our laboratories and is likely a critical target for new approaches to enhance SPM and other resolution pharmacology-based therapies.
In addition to inflammatory exudates, lipid mediator class switching (63) illustrated in Figure 1 occurs within reparative human M2-like macrophages ((71), Table 1). These results, now from several independent laboratories, point to a specific proresolving phospholipase A2 (58, 66) that can hydrolyze phospholipid esterified EPA and esterified DHA to their free unesterified forms that are next available for conversion to proresolving mediators (Fig. 1). These specific phospholipases can now be targeted for new pro-resolving therapies to control local inflammation in the respiratory system.
Thus, self-limited resolving exudates biosynthesize SPM in a temporally orchestrated manner that coincides with leukocyte traffic to the site of inflammation and their transcellular biosynthesis of key substrates and intermediates (Figure 1).
These include the different types of inflammatory exudates, namely:
Serous exudates:
defined as few in leukocyte numbers, yellowish-colored fluid, containing protein and fibrin-free serum, e.g. “transudative” pleural fluid with pleural fluid/serum total protein < 0.5, lactate dehydrogenase ratio < 0.6, and pleural fluid LDH < (2/3 *upper limit of normal serum LDH) (63).
Purulent exudates:
Infectious, high numbers of leukocytes and cellular debris, creamy white-or green-colored fluid (72).
Hemorrhagic exudates:
can be sterile or infectious; contains leukocytes and red blood cells with red or brown-colored fluid (73).
The presence of large numbers of red blood cells in the exudate indicates vascular damage from infarction, infection, or tumors with excessive leukocyte extravasation contributing to lung tissue damage. Exudates temporally biosynthesize the SPMs to promote tissue repair and the return this injured tissue to a new functional homeostasis.
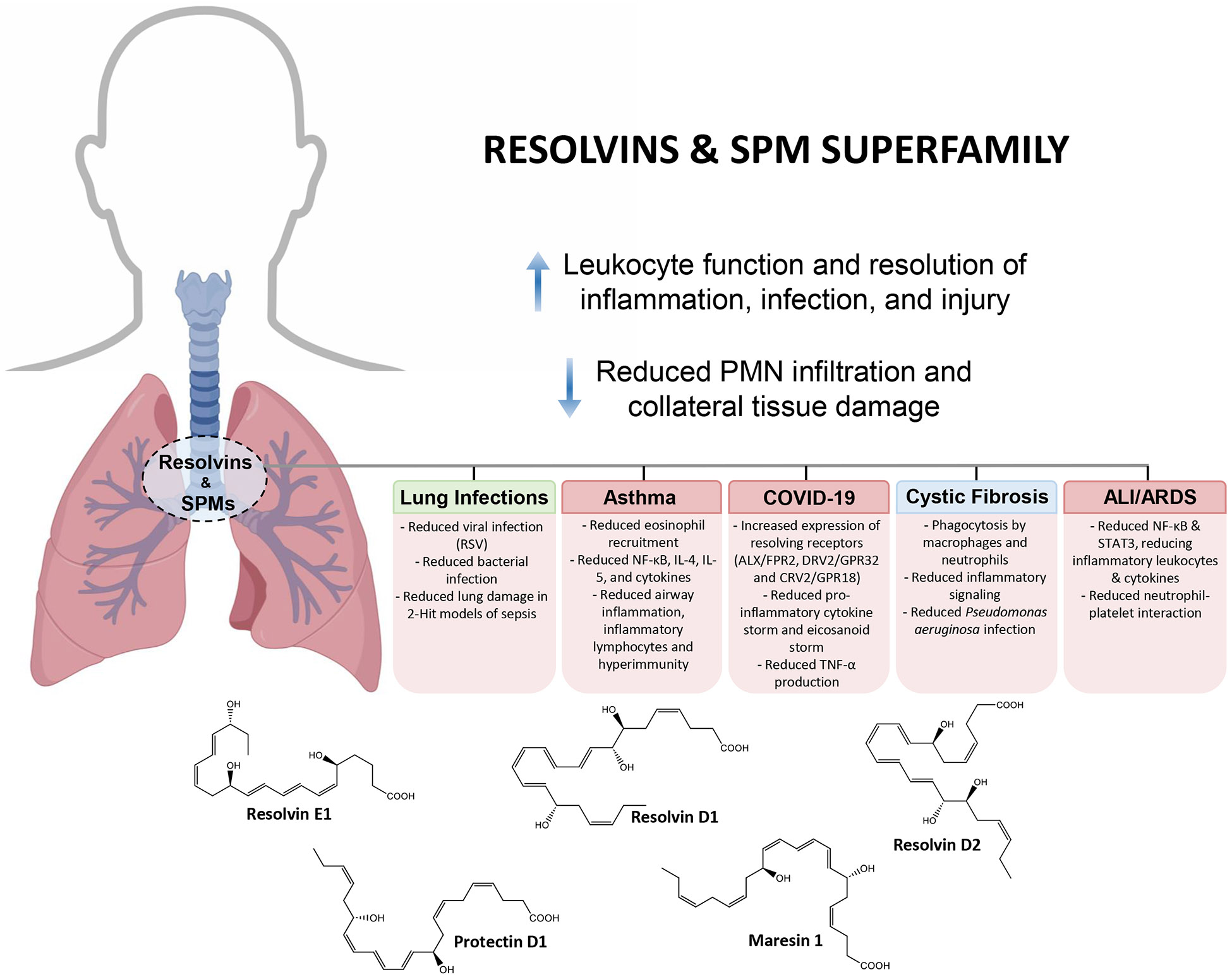
As the rigorous large-scale human clinical trials with resolvin and SPM biosynthetic precursors yield results indicating improved lung functions (7), the interest in SPM and biosynthetic mechanisms to increase their endogenous production is heightened. Immunoresolvents such as the natural combination medicine Traumeel (Tr14) stimulate the biosynthesis of SPMs in exudates in peritonitis and by human M2-like macrophages activated with Staphylococcus aureus (74). Opportunities to increase wellness and resilience were recently noted by the NIH (75, 76). The therapeutic potential of resolvins in pulmonary diseases was also recently the subject of an independent review (23). The elucidation of the resolvins and other SPM has led to a shift in thinking about treating airway and lung inflammation and infection with molecules that possess the capacity to control of excessive inflammation. The pro-resolving and host-protective actions of the resolvins and SPM (77) are of special interest in the respiratory system (72, 78-80), given that these endogenous mediators can be functionalized to serve as precision therapeutics in airway diseases, for example, the 17R-Resolvin D1 analog 17R-hydroxy-19-para-fluorophenoxy-Resolvin D1 (70, 77, 81) and others including the benzo-resolvin D1 (82, 83).
The SPMs may also have a role in protecting the lung from pulmonary fibrosis (43). This is of urgent special interest because currently there are limited therapeutic options for pulmonary fibrosis as seen in interstitial lung disease, idiopathic pulmonary fibrosis, and sarcoidosis pathogenesis (84, 85) . Administration of SPMs has the potential to prevent transition from inflammation/lung injury to lung fibrosis and respiratory failure (19). The proresolving signaling of the SPM is an advantage in these lung diseases (Table 1).
Human Lung and SPMs
Since the first evidence for leukotrienes in asthma and inflammation appeared in 1989 (86), the human respiratory system has been widely studied and recognized as a substantial source of lipid mediators. Indeed, recent analysis of human lung tissues from human lung grafts in transplant patients, both prereperfusion and postreperfusion, clearly demonstrated, using targeted LC-MS-MS-based methods, that human lung tissue produced substantial amounts of 17-HDHA and D-series resolvins in amounts well within their potent bioactive concentrations on human leukocytes (70, 87). These were found in the low subnanomolar range. While the amounts of resolvins in humans were questioned as enough to evoke their potent pro-resolving actions, Fu et al. independently reported that both human plasma and mouse tissues contain lipoxins and resolvins identified in the picogram range, in line with their potent stereoselective immunoresolving functions (25).
These were also independently confirmed recently by Zhu et al. (88) using targeted LC-MS-MS together with a derivatization method that documented Resolvin D1, Resolvin D2, Resolvin E3 and lipoxins in sera from healthy volunteers. Earlier results clearly demonstrated that resolvin and SPM production in human serum and plasma was dependent on the amounts of EPA and DHA taken by healthy volunteers (89, 90). Thus, if adequate substrates of EPA and DHA are ingested and present, Resolvin D1 is biosynthesized, which can accelerate the resolution of inflammation (Figure 1B) and reduce the impact of acute lung injury (91). Extracellular vesicles are a source of increased SPM substrate availability and biosynthetic enzymes that in inflamed respiratory tissues can locally produce SPM (68, 92).
We next shall consider the new evidence for SPM in human airway and lung disease and specific animal lung disease models.
Chronic rhinosinusitis.
In a recent study with human subjects, Resolvin D2 was elevated in subjects with polyps; both resolvin D1 and resolvin D2 are diminished in smokers compared to non-smokers. Using LC-MS-MS based detection, several SPM correlated with sinonasal mucosal microbiota as well, as with pathogens such as Pseudomonas aeruginosa (31), including LXA4, LXB4 and resolvins (RvD1, RvD2, RvD3, RvD5 and RvE1). The presence of protectins (PDX) and MaR1 in nasal polyps and nasal mucosa have also been recently examined. SPM concentrations were significantly higher in the control subjects without chronic inflammation (93).
Cystic fibrosis.
The airway and lung inflammation in cystic fibrosis (CF) are classic examples (Figure 1B) of non-resolving inflammation in upper and lower human airways. Sputum from CF patients analyzed using LC-MS-MS-based methods documents the proresolving mediators resolvin E1 and Lipoxin A4 among many proinflammatory mediators, e.g. LTB4, PGE2 and PGD2 (94). Prof. Hammock and colleagues point out that patients with detectable resolvin E1 had better lung function than patients with low or undetectable RvE1 in their sputum (94), demonstrating the utility of lipid mediator-metabololipidomic profiling in CF sputum, which may be useful in temporal analysis of a broader range of human pathologic and disease processes (95). Of interest, P. aeruginosa, a key pathogen in CF, can disrupt proresolving lipid mediators (96). Eickmeier et al. demonstrated that, in CF, the D-series Resolvin D1 is a potential marker of lung disease (34). When Resolvin D1 is given as a therapeutic in an animal CF model, Resolvin D1 stimulates resolution of lung inflammation (97) and is a potent broncho-protective SPM (98).
Urbach and colleagues found that in CF patients the airway epithelium plays significant roles in SPM biosynthesis and actions (99) and is sex dependent (35, 100, 101). Both airway inflammation and exercise capacity in CF are improved with resolvin D1 (32, 102, 103).
Macrophages from CF patients exposed to SARS-CoV-2 elaborate a cytokine-chemokine storm that is reduced and resolved with Resolvin D1 and Resolvin D2 (33). Thornton et al. (104) discovered that lipoxin A4 in biofilm improves antibiotic efficacy with P. aeruginosa, suggesting that the SPMs, which can activate endogenous resolution mechanisms, can help reduce chronic inflammation and infections in the respiratory tract.
Respiratory tract infection and pneumonia.
Several recent publications have provided insights regarding mechanisms for augmentation of airway and lung host defense by SPM. In a mouse model of Escherichia coli pneumonia, lung SPM levels were temporally regulated, and early treatment with exogenous AT-RvD1 (1 h post infection) enhanced clearance of E. coli and P. aeruginosa in vivo, increasing expression of anti-microbial peptides and accelerating lung macrophage phagocytosis of bacteria (14, 105). AT-RvD1 also increased efferocytosis by infiltrating macrophages (CD11bHi CD11cLow) and exudative macrophages (CD11bHi CD11cHi), and enhanced neutrophil clearance during pneumonia in vivo. Importantly, these anti-bacterial and pro-resolving actions of AT-RvD1 were additive to antibiotic therapy (14).
Lung macrophages are also targeted by the cysteinyl-maresins to augment host defense. Serious respiratory viral infection (e.g., from influenza) can decrease alveolar macrophage numbers and reprogram the cells to promote inflammation. Harnessing endogenous macrophage resolution mechanisms for inflammation with exogenous cysteinyl-maresins increases macrophage resilience after influenza to protect against secondary bacterial pneumonia from Streptococcus pneumoniae (106). Select SPMs serve as endogenous agonists for A20 and single Ig IL-1R-related molecule (SIGIRR) expression to regulate macrophage and airway epithelial cell NF-κB activity as mechanisms for control of lung inflammation and pneumonia resolution (105).
Respiratory syncytial virus (RSV) is a major respiratory pathogen with excess morbidity in early life and older individuals. The morbidity and mortality associated with RSV is secondary to an exacerbated host immune responses and injury to the epithelium that can obstruct the airway and compromise gas exchange. In a mouse model of RSV infection from a human clinical strain (i.e., Line 19), lung PD1 and PCTR1 levels were temporally regulated, and their exogenous administration three days after inoculation enhanced viral clearance and promoted resolution of the host immune response, in part secondary to increased epithelial interferon-lambda and decreased CD4 effector T cell interferon-gamma (107). In addition, MaR1 signaling via its recently identified receptor Lgr6 improves regulatory T cell suppressive function for T cell cytokine production and upregulates host antiviral genes and amphiregulin production to decrease viral burden and pathogen-evoked inflammation, highlighting important roles for SPM-informed regulatory T cells in the restitution of disrupted airway mucosal homeostasis (108). The most serious host response to lung infection is sepsis. Of interest, recent cellular phenotyping from critically ill human patients identified that RvD1 and RvD2 signaling for antiinflammation and resolution are uncoupled from leukocyte activation in early sepsis; findings that point to diminished resolution signaling as a correlate of clinical disease severity (109).
Asthma and allergic lung inflammation.
Asthma is the most common respiratory illness, and immunophenotyping of most patients reveals chronic type 2 inflammation with increased pro-phlogistic mediators, prominent eosinophilia, bronchial epithelial mucous cell metaplasia and airway hyperresponsiveness (110). Asthma control therapy currently emphasizes anti-inflammatory agents, including corticosteroids and type 2 pathway targeted biologics (111); however, these therapies are not curative, failing to fully resolve asthma.
Recent findings have uncovered several intriguing pro-resolving roles for SPM in allergic lung inflammation and asthma. In a DRV2 receptor-dependent manner, resolvin D2 (RvD2) accelerates resolution of mouse lung inflammation evoked by house dust mite sensitization and challenge, and DRV2 receptors are expressed on eosinophils and other leukocytes (112). Eosinophils are heterogeneous with two subsets in mice that are identifiable by CD101 expression with distinct anatomic localization and transcriptional signatures at baseline and during lung inflammation (113). CD101low Eos are predominantly in a vascular niche and respond to airway allergen challenge by trafficking into the lung interstitium where they acquire an activated phenotype with high expression of CD101 and continued trafficking into the airways where they are detectable in whole lung lavage. RvD2 reduces total Eos numbers and activation by decreasing interleukin 5-dependent lung recruitment of CD101low Eos and decreasing eosinophil conversion from CD101low to CD101high (113).
Cysteinyl leukotrienes (CysLTs) have been assigned important roles in asthma pathophysiology as potent pro-phlogistic mediators; however, inhibition of CysLT1 receptors has not proven consistently effective as a therapeutic strategy. Additional regulatory mechanisms were recently identified to address this gap in knowledge. Cysteinyl-containing lipid mediators derived from DHA, namely the maresin conjugates of tissue regeneration (MCTRs), are produced in human and mouse lung and can block human LTD4-induced airway contraction and promote resolution in vivo of mouse allergic airway responses (39); endogenous mechanisms for control of asthma phenotypes.
In addition to eosinophil activation, the immunology of the severe asthma airway is notable for decreased NK cell cytotoxicity with increased numbers of NK cell targets, such as granulocytes and effector lymphocytes, which is exacerbated by corticosteroids that further disable NK cell function (114). In contrast to corticosteroids, the SPM LXA4 preserved NK cell functional responses (114). The LXA4 receptor ALX/FPR2 can engage both proresolving and proinflammatory ligands for opposing signaling events, enabling these receptors to serve pivotal roles in the fate of lung inflammatory responses. In bronchoalveolar lavage (BAL) fluid, levels of LXA4 and 15-epi-LXA4 are decreased, and the acute phase reactant serum amyloid A (SAA) is increased in severe asthma relative to non-severe asthma (115). These select ALX receptor ligands define a biochemical endotype for asthma as patients with LXA4loSAAhi levels have characteristics associated with severe asthma, namely increased BAL neutrophils, more asthma symptoms, lower lung function, and increased relative risk for asthma exacerbation, sinusitis, and gastroesophageal reflux disease (115).
Acute respiratory distress syndrome.
Perhaps the most devastating lung disease is the acute respiratory distress syndrome (ARDS). Acute inflammation fills the gas exchanging alveoli, leading to life threatening hypoxemia, so patients most commonly require supplemental oxygen via mechanical ventilation. Of interest, lower plasma levels of SPM are associated with increased duration of ventilatory support and ICU length of stay (116). Both plasma LXA4 and MaR1 levels are decreased in ARDS (116) and these SPM are protective in experimental acute lung injury in mice (117, 118). These SPM can be produced by circulating leukocyte-platelets aggregates, which are prominent in acute inflammation, augment lung leukocyte infiltration via secondary capture, and are associated with incident human ARDS (119), suggesting that the diminished restraining activities from the lower plasma SPM levels unleash leukocyte activation and transmigration into the lung in ARDS and that exogenous SPM administration could represent a new therapeutic strategy for ARDS.
SPM Therapeutic Potential for Airway Inflammation in Pulmonary Diseases
Given the potent proresolving actions of each SPM demonstrated in many animal disease models, several of the SPMs and their cognate receptors are being considered for new therapeutic approaches in pulmonary diseases (23, 120). This shift in the search for new proresolving therapies from the current immune suppression of pro-inflammatory mediators and pathways is based on the potent stereospecific actions of the SPMs in regulating neutrophil migration for lung protection (87, 121-123), enhancing bacterial and viral clearance (14, 106-108, 123), reducing cytokine and eicosanoid storms (105), stimulating phagocytosis of apoptotic PMNs (efferocytosis), removal of debris, and reducing pain to shorten resolution intervals (Ri) (78).
Following the total organic synthesis and assignment of the complete stereochemistry of each of the first endogenous SPMs originally uncovered to confirm their potent proresolving and anti-inflammatory functions (reviewed in 124), additional total syntheses of specific SPMs have been achieved, for example, refs. (125-129). These enabled further investigations of in vivo SPM functions in animal models and identification of specific proresolving receptors. At this point, five proresolving receptors are identified (illustrated in Figure 2) along with their Kd and concentration range to evoke pro-resolving phagocytosis; for RvD1: ALX/FPR2, human GPR-32 (130-132); for RvE1: ChemR23 (45); RvD2: GPR18 (41); and MaR1: LGR6 (reviewed in 108, 133). Several of these receptors were further validated in receptor knockout mice (134) as for the Resolvin D2 receptor (38, 133) and MaR1 receptor (108).
As part of their intracellular signaling, the SPMs induce heme oxygenase-1 (HO-1) (135), which produces carbon monoxide. Inhaled CO is organ protective in the lung, demonstrated in ischemia-reperfusion lung injury (136). Low-dose inhaled CO (125-250 ppm) reduces PMN-platelet aggregates and reduces PMN infiltration into the lung. Both resolvin D1 and low-dose inhaled CO reduce leukocyte-mediated acute lung injury (122, 136, 137). SPMs restore barrier integrity (122, 138). In baboon pneumonia with S. pneumoniae, prostaglandins and leukotriene B4 (LTB4) are increased. Inhaled low-dose CO reduced these proinflammatory eicosanoids and increased SPM, which may shorten the time to resolve pneumonia (37). These results suggest that therapies that increase endogenous SPM may have a beneficial impact on the lung during acute injury or infection. Along these lines, extracts of Hyssopus Cuspidatus Boriss are used to treat asthma in some parts of the world, and these plant extracts have been shown to increase both resolvin and lipoxin production in mice with experimental allergic inflammation using UHPLC-MS-MS for identification and quantitation, suggesting an SPM mechanism of action for this ancient therapy (139). The newly described peptide-containing SPM, namely the cys-SPM (Figure 3) such as MCTR1 (Figure 3), are also lung protective in experimental animal models of lung inflammation (40). Traumeel (Tr14) stimulates SPM biosynthesis, improving the resolution of inflammation in mouse peritonitis (74).
Figure 3. Biosynthesis Pathways for the cys-SPMs and SPMs.

Panel A) The Proposed Biosynthesis of Maresins and cys-Maresins. The complete stereochemistry of each structure shown was established by total organic synthesis and functions confirmed for each potent bioactive molecule of the specialized proresolving mediator superfamily of novel molecules. For examples, see Serhan et al. (61); Dalli et al. (156, 157); and Serhan (62).
Panel B) Biosynthesis proposed for the Protectins and cys-Protectins (PCTRs). The stereochemistry of NPD1/PD1 was established in Serhan et al. (158); Ramon et al. (159); and de la Rosa et al. (81) employing materials prepared by total organic synthesis with each validated using NMR.
Panel C) Proposed Biosynthesis of the cys-Resolvins (RCTRs) and relation to D-Series Resolvin. The stereochemistry of RCTRs 1, 2 and 3 were established; see Ref. (81), and potent actions confirmed with defined synthetic materials prepared by total organic synthesis (81).
Lipid emulsions, which are widely used in critical care medicine as part of total parenteral nutrition, increase the biosynthesis of SPM in human immune cells and mouse tissues (26). In asthmatic patients, 17-HDHA and Resolvin D5 are produced by nasal epithelium, which may be useful to stage the severity of asthma in these patients (140). Indeed, SPM are present in lungs of intubated COVID-19 patients (141) in substantial and sustained amounts.
SPMs are also demonstrable in plasma of human subjects with chronic inflammation (142, 143) and arthritis (29), which increase with EPA and DHA supplementation (89). The identification of SPM in human disease and their increase with supplementation suggest a functional role for SPM in shortening recovery and return to homeostasis (49). To demonstrate the potential of SPM-based resolution therapies, metabolically stable analogs of Resolvin E1 and Resolvin D1 were designed and synthesized; each was demonstrated in vivo in mice to be highly effective (51, 82), giving proof to the new concept that stimulating resolution of inflammation with SPM mimetics and related pro-resolution approaches can be a new line of treatment for airway and lung infections and inflammation.
During studies to map the mechanisms and mediators involved in the resolution of inflammation, we found that the vagus nerve controls resolution of inflammation by stimulating the production of pro-resolving mediators (144) using the vagotomy approach introduced by K. Tracey and colleagues (144, 145). Electrical stimulation of the human vagus ex vivo inhibits the vagus nerve production of prostaglandins and leukotrienes while stimulating the production of pro-resolving mediators, i.e. SPMs (36). Vagus nerve stimulation of SPM production was confirmed by Huang et al. (146), which also demonstrated reduced lung permeability in acute lung injury in mice. Thus, electrical stimulation of the vagus nerve to produce SPM can be a further treatment option in airway inflammation-associated pulmonary diseases. The discovery that dexamethasone can stimulate SPM production in M2-like macrophages and in humans with COVID-19 infections opens many new opportunities to repurpose dexamethasone and related drugs to new SPM-based pro-resolving therapies (147) to bring in resolution pharmacology (78).
Conclusions and Summary
We’ve reviewed herein recent contributions on the Resolvins, Protectins and Maresins focusing on their functions in the respiratory system. Collectively, these endogenous mediators of resolution are referred to as the SPM superfamily of lipid mediators. For this annual review we’ve focused on SPM in the lung/respiratory system with substantial and far-reaching contributions as updates since our earlier annual review on these novel molecules in the physiology of inflammation-resolution lung tissues (17). The SPMs demonstrate proresolving functions independently confirmed by many, and their potent pro-resolution functions and criteria are established as follows a) reduction of pro-inflammatory cytokines and eicosanoid storm, b) enhanced killing, c) clearance of bacteria, d) increased efferocytosis by macrophages, e) removal of cellular debris by phagocytosis, f) reducing the resolution/recovery time interval (Ri), and g) reducing pain. These features and endogenous functions of the SPM are attractive for airway diseases and potentially now provide a wealth of evidence to substantiate a shift from inhibitors – receptor antagonists to exciting new directions for treatment of respiratory disease using activators – receptor agonists of endogenous resolution mechanisms to control excessive inflammation and infections in the respiratory system.
The respiratory tract mucosa is enriched in omega 3 fatty acids EPA and DHA in health, and tissue stores of these SPM substrates decrease in inflammatory disease (148). Local production of the SPMs is lung protective in both vascular and extravascular niches, and their abundance or signaling mechanisms are disrupted in acute and chronic disease. As physiologic counter-regulatory modulators, several experimental systems have provided evidence that the SPMs and cysteinyl-SPMs instruct lung tissue resident cells and macrophages to recapture homeostasis and organ function. We eagerly anticipate the next horizon in resolution biology and pharmacology that will see new therapeutic modalities emerge that leverage the SPM endogenous protective and tissue regenerative mechanisms for resolution medicines to ameliorate the excess morbidity and mortality of acute and chronic diseases of the respiratory tract.
Acknowledgments:
The authors gratefully acknowledge support from the NIH 1R35GM139430 (CNS), 1U01HL146002 (BDL), 1R01HL122531 (BDL), R21GM144829 (BDL), 1R56ES033250 (CNS, BDL), and R01HL168899 (CNS, BDL) for support of our research programs. Figures 2 and 3 were created with BioRender.com . We also thank Mary Small for expert assistance in manuscript preparation.
Literature Cited
- 1.Samuelsson B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 220: 568–75 [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. 1987. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237: 1171–76 [DOI] [PubMed] [Google Scholar]
- 3.Haeggström JZ, Newcomer ME. 2023. Structures of Leukotriene Biosynthetic Enzymes and Development of New Therapeutics. Annu Rev Pharmacol Toxicol 63: 407–28 [DOI] [PubMed] [Google Scholar]
- 4.Calder PC. 2020. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 178: 105–23 [DOI] [PubMed] [Google Scholar]
- 5.Soehnlein O, Libby P. 2021. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov 20: 589–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carracedo M, Artiach G, Arnardottir H, Bäck M. 2019. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin Immunopathol 41: 757–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patchen BK, Balte P, Bartz TM, Barr RG, Fornage M, et al. 2023. Investigating Associations of Omega-3 Fatty Acids, Lung Function Decline, and Airway Obstruction. Am J Respir Crit Care Med 208: 846–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eivazi A, Akbari B, Falahi S, Gorgin Karaji A, Rezaiemanesh A, et al. 2023. Association of Rs7217186 Polymorphism of Arachidonic Acid 15-Lipoxygenase (ALOX15) Gene with Susceptibility to Allergic Rhinitis. Rep Biochem Mol Biol 12: 269–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, et al. 2016. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med 375: 2530–9 [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Hu FB, Manson JE. 2019. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J Am Heart Assoc 8: e013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins SL, Cotran R. 1979. Pathologic Basis of Disease. Philadelphia: W.B. Saunders Co. 1598 pp. [Google Scholar]
- 12.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med 192: 1197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN, Savill J. 2005. Resolution of inflammation: the beginning programs the end. Nat. Immunol 6: 1191–97 [DOI] [PubMed] [Google Scholar]
- 14.Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, et al. 2016. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol 9: 1278–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan CN. 2007. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu. Rev. Immunol 25: 101–37 [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Yacoubian S, Yang R. 2008. Anti-inflammatory and pro-resolving lipid mediators. Annu. Rev. Pathol. Mech. Dis 3: 279–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy BD, Serhan CN. 2014. Resolution of acute inflammation in the lung. Annu. Rev. Physiol 76: 27.1–27.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang MJ, Spite M. 2012. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu. Rev. Nutr 32: 203–27 [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN, Levy BD. 2018. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 128: 2657–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perretti M, Dalli J. 2023. Resolution Pharmacology: Focus on Pro-Resolving Annexin A1 and Lipid Mediators for Therapeutic Innovation in Inflammation. Annu Rev Pharmacol Toxicol 63: 449–69 [DOI] [PubMed] [Google Scholar]
- 21.Godson C, Guiry P, Brennan E. 2023. Lipoxin Mimetics and the Resolution of Inflammation. Annu Rev Pharmacol Toxicol 63: 429–48 [DOI] [PubMed] [Google Scholar]
- 22.Ji RR. 2023. Specialized Pro-Resolving Mediators as Resolution Pharmacology for the Control of Pain and Itch. Annu Rev Pharmacol Toxicol 63: 273–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centanni D, Henricks PAJ, Engels F. 2023. The therapeutic potential of resolvins in pulmonary diseases. Eur J Pharmacol: 176047. [DOI] [PubMed] [Google Scholar]
- 24.Daly K, O'Sullivan K, O'Sullivan TP. 2022. Major structure-activity relationships of resolvins, protectins, maresins and their analogues. Future Med Chem 14(24): 1943–60 [DOI] [PubMed] [Google Scholar]
- 25.Fu X, Yin HH, Wu MJ, He X, Jiang Q, et al. 2022. High Sensitivity and Wide Linearity LC-MS/MS Method for Oxylipin Quantification in Multiple Biological Samples. J Lipid Res 63: 100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noureddine N, Hartling I, Wawrzyniak P, Srikanthan P, Lou PH, et al. 2022. Lipid emulsion rich in n-3 polyunsaturated fatty acids elicits a pro-resolution lipid mediator profile in mouse tissues and in human immune cells. Am J Clin Nutr 116: 786–97 [DOI] [PubMed] [Google Scholar]
- 27.Hartling I, Cremonesi A, Osuna E, Lou PH, Lucchinetti E, et al. 2021. Quantitative profiling of inflammatory and pro-resolving lipid mediators in human adolescents and mouse plasma using UHPLC-MS/MS. Clin Chem Lab Med 59: 1811–23 [DOI] [PubMed] [Google Scholar]
- 28.Barden A, Shinde S, Beilin LJ, Phillips M, Adams L, et al. 2024. Adiposity associates with lower plasma resolvin E1 (Rve1): a population study. Int J Obes (Lond) doi: 10.1038/s41366-024-01482-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. 2016. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids 107: 24–29 [DOI] [PubMed] [Google Scholar]
- 30.Schwarz B, Sharma L, Roberts L, Peng X, Bermejo S, et al. 2021. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J Immunol 206: 329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vickery TW, Armstrong M, Kofonow JM, Robertson CE, Kroehl ME, et al. 2021. Altered tissue specialized pro-resolving mediators in chronic rhinosinusitis. Prostaglandins Leukot Essent Fatty Acids 164: 102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isopi E, Mattoscio D, Codagnone M, Mari VC, Lamolinara A, et al. 2020. Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis. Front Immunol 11: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recchiuti A, Patruno S, Mattoscio D, Isopi E, Pomilio A, et al. 2021. Resolvin D1 and D2 reduce SARS-CoV-2-induced inflammatory responses in cystic fibrosis macrophages. FASEB J 35: e21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eickmeier O, Fussbroich D, Mueller K, Serve F, Smaczny C, et al. 2017. Pro-resolving lipid mediator Resolvin D1 serves as a marker of lung disease in cystic fibrosis. PLoS One 12: e0171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briottet M, Shum M, Urbach V. 2020. The Role of Specialized Pro-Resolving Mediators in Cystic Fibrosis Airways Disease. Front Pharmacol 11: 1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan CN, De la Rosa X, Jouvene CC. 2018. Cutting Edge: Human vagus produces specialized pro-resolving mediators of inflammation with electrical stimulation reducing pro-inflammatory eicosanoids. J. Immunol 201: 3161–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalli J, Kraft BD, Colas RA, Shinohara M, Fredenburgh LE, et al. 2015. The Regulation of Proresolving Lipid Mediator Profiles in Baboon Pneumonia by Inhaled Carbon Monoxide. Am J Respir Cell Mol Biol 53: 314–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serhan CN, Chiang N. 2023. Resolvins and cysteinyl-containing pro-resolving mediators activate resolution of infectious inflammation and tissue regeneration. Prostaglandins Other Lipid Mediat 166: 106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy BD, Abdulnour RE, Tavares A, Brüggemann TR, Norris PC, et al. 2020. Cysteinyl maresins regulate the prophlogistic lung actions of cysteinyl leukotrienes. J Allergy Clin Immunol 145: 335–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godson C. 2020. Balancing the Effect of Leukotrienes in Asthma. N Engl J Med 382: 1472–75 [DOI] [PubMed] [Google Scholar]
- 41.Chiang N, Dalli J, Colas RA, Serhan CN. 2015. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 212: 1203–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trilleaud C, Gauttier V, Biteau K, Girault I, Belarif L, et al. 2021. Agonist anti-ChemR23 mAb reduces tissue neutrophil accumulation and triggers chronic inflammation resolution. Sci Adv 7: eabd1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thatcher TH, Freeberg MAT, Myo YPA, Sime PJ. 2023. Is there a role for specialized pro-resolving mediators in pulmonary fibrosis? Pharmacol Ther 247: 108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fredman G, Serhan CN. 2024. Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease. Nat Rev Cardiol doi: 10.1038/s41569-023-00984-x [DOI] [PubMed] [Google Scholar]
- 45.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, et al. 2005. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med 201: 713–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, et al. 2006. Resolvin E2: Identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol 13: 1193–202 [DOI] [PubMed] [Google Scholar]
- 47.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. 2011. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest 121: 569–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cebrián-Prats A, Pinto A, González-Lafont À, Fernandes PA, Lluch JM. 2022. The role of acetylated cyclooxygenase-2 in the biosynthesis of resolvin precursors derived from eicosapentaenoic acid. Org Biomol Chem 20: 1260–74 [DOI] [PubMed] [Google Scholar]
- 49.Serhan CN, Libreros S, Nshimiyimana R. 2022. E-series resolvin metabolome, biosynthesis and critical role of stereochemistry of specialized pro-resolving mediators (SPMs) in inflammation-resolution: Preparing SPMs for long COVID-19, human clinical trials, and targeted precision nutrition. Semin Immunol 59: 101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. 2012. Resolvin E2 formation and impact in inflammation resolution. J. Immunol 188: 4527–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arita M, Oh S, Chonan T, Hong S, Elangovan S, et al. 2006. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem 281: 22847–54 [DOI] [PubMed] [Google Scholar]
- 52.Norris PC, Libreros S, Serhan CN. 2019. Resolution metabolomes activated by hypoxic environment. Sci. Adv 5: eaax4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libreros S, Shay AE, Nshimiyimana R, Fichtner D, Martin MJ, et al. 2021. A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front Immunol 11: 631319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reinertsen AF, Primdahl KG, Shay AE, Serhan CN, Hansen TV, Aursnes M. 2021. Stereoselective Synthesis and Structural Confirmation of the Specialized Pro-Resolving Mediator Resolvin E4. J Org Chem 86: 3535–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, et al. 2002. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med 196: 1025–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, et al. 2014. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med 211: 1673–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, et al. 2014. The Specialized Proresolving Mediator 17-HDHA Enhances the Antibody-Mediated Immune Response against Influenza Virus: A New Class of Adjuvant? J Immunol 193: 6031–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J. Biol. Chem 278: 14677–87 [DOI] [PubMed] [Google Scholar]
- 59.Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, et al. 2018. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. 2015. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 1851: 397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, et al. 2009. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J. Exp. Med 206: 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, et al. 2012. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26: 1755–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. 2001. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol 2: 612–19 [DOI] [PubMed] [Google Scholar]
- 64.Dahlke P, Peltner LK, Jordan PM, Werz O. 2023. Differential impact of 5-lipoxygenase-activating protein antagonists on the biosynthesis of leukotrienes and of specialized pro-resolving mediators. Front Pharmacol 14: 1219160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Röhn TA, Numao S, Otto H, Loesche C, Thoma G. 2021. Drug discovery strategies for novel leukotriene A4 hydrolase inhibitors. Expert Opin Drug Discov 16: 1483–95 [DOI] [PubMed] [Google Scholar]
- 66.Miki Y, Yamamoto K, Taketomi Y, Sato H, Shimo K, et al. 2013. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J Exp Med 210: 1217–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norling LV, Dalli J. 2013. Microparticles are novel effectors of immunity. Curr Opin Pharmacol 13: 570–5 [DOI] [PubMed] [Google Scholar]
- 68.Dalli J, Serhan CN. 2012. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120: e60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, et al. 2005. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest 115: 2774–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, et al. 2008. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol 181: 8677–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. 2014. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci U S A 111: 12746–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery TW, et al. 2012. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484: 524–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norris PC, Libreros S, Chiang N, Serhan CN. 2017. A cluster of immunoresolvents links coagulation to innate host defense in human blood. Sci Signal 10: eaan1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jordan PM, van Goethem E, Müller AM, Hemmer K, Gavioli V, et al. 2021. The Natural Combination Medicine Traumeel (Tr14) Improves Resolution of Inflammation by Promoting the Biosynthesis of Specialized Pro-Resolving Mediators. Pharmaceuticals (Basel) 14: 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor HA, Finkel T, Gao Y, Ballinger SW, Campo R, et al. 2022. Scientific opportunities in resilience research for cardiovascular health and wellness. Report from a National Heart, Lung, and Blood Institute workshop. FASEB J 36: e22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scruggs S. 2019. Inflammation resolution gets top billing at NIH workshop. Environmental Factor https://factor.niehs.nih.gov/2019/5; accessed 2023/09/25 [Google Scholar]
- 77.Tang H, Liu Y, Yan C, Petasis NA, Serhan CN, Gao H. 2014. Protective actions of aspirin-triggered (17R) resolvin D1 and its analogue, 17R-hydroxy-19-para-fluorophenoxy-resolvin D1 methyl ester, in C5a-dependent IgG immune complex-induced inflammation and lung injury. J Immunol 193: 3769–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510: 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee CR, Zeldin DC. 2015. Resolvin Infectious Inflammation by Targeting the Host Response. N Engl J Med 373: 2183–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basil MC, Levy BD. 2016. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16: 51–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de la Rosa X, Norris PC, Chiang N, Rodriguez AR, Spur BW, Serhan CN. 2018. Identification and complete stereochemical assignments of the new Resolvin Conjugates in Tissue Regeneration (RCTR) in human tissues that stimulate proresolving phagocyte functions and tissue regeneration. Am J Pathol. 188: 950–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orr SK, Colas RA, Dalli J, Chiang N, Serhan CN. 2015. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am J Physiol Lung Cell Mol Physiol 308: L904–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murakami Y, Fukuda H, Muromoto R, Hirashima K, Ishimura K, et al. 2020. Design and Synthesis of Benzene Congeners of Resolvin E2, a Proresolving Lipid Mediator, as Its Stable Equivalents. ACS Med Chem Lett 11: 479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savin IA, Zenkova MA, Sen'kova AV. 2022. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int J Mol Sci 23: 14959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee TH, Crea AE, Gant V, Spur BW, Marron BE, et al. 1990. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. American Review of Respiratory Disease 141: 1453–8 [DOI] [PubMed] [Google Scholar]
- 86.Dahlén S-E, Dahlén B, Kumlin M, Björck T, Ihre E, Zetterström O. 1994. Clinical and experimental studies of leukotrienes as mediators of airway obstruction in humans. Adv. Prost. Thrombox. Leukot. Res 22: 155–66 [PubMed] [Google Scholar]
- 87.Li W, Shepherd HM, Terada Y, Shay AE, Bery AI, et al. 2023. Resolvin D1 prevents injurious neutrophil swarming in transplanted lungs. Proc Natl Acad Sci U S A 120: e2302938120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu H, Kurokawa M, Chen M, Wang Q, Inoue M, Takao T. 2023. Characteristic fragmentation of polyunsaturated fatty acids with allylic vicinal diols in positive-ion LC/ESI-MS/MS. J Lipid Res 64: 100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mas E, Croft KD, Zahra P, Barden A, Mori TA. 2012. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem 58: 1476–84 [DOI] [PubMed] [Google Scholar]
- 90.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. 2014. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol 307: C39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiang SY, Ye Y, Yang Q, Xu HR, Shen CX, et al. 2021. RvD1 accelerates the resolution of inflammation by promoting apoptosis of the recruited macrophages via the ALX/FasL-FasR/caspase-3 signaling pathway. Cell Death Discov 7: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Devitt A, Griffiths HR, Milic I. 2018. Communicating with the dead: lipids, lipid mediators and extracellular vesicles. Biochem Soc Trans 46: 631–39 [DOI] [PubMed] [Google Scholar]
- 93.Zhu ZZ, Wang WQ, Han JB, Wang L, Lyu W. 2021. [Different concentrations of specialized pro-resolving mediators in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 56: 1073–79 [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. 2012. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med 53: 160–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norris PC, Serhan CN. 2018. Metabololipidomic profiling of functional immunoresolvent clusters and eicosanoids in mammalian tissues. Biochem Biophys Res Commun 504: 553–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, et al. 2017. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci U S A 114: 136–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Codagnone M, Cianci E, Lamolinara A, Mari VC, Nespoli A, et al. 2018. Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal Immunol 11: 35–49 [DOI] [PubMed] [Google Scholar]
- 98.Duvall MG, Bruggemann TR, Levy BD. 2017. Bronchoprotective mechanisms for specialized pro-resolving mediators in the resolution of lung inflammation. Mol Aspects Med 58: 44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ringholz FC, Higgins G, Hatton A, Sassi A, Moukachar A, et al. 2018. Resolvin D1 regulates epithelial ion transport and inflammation in cystic fibrosis airways. J Cyst Fibros 17: 607–15 [DOI] [PubMed] [Google Scholar]
- 100.Shum M, London CM, Briottet M, Sy KA, Baillif V, et al. 2022. CF Patients' Airway Epithelium and Sex Contribute to Biosynthesis Defects of Pro-Resolving Lipids. Front Immunol 13: 915261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Philippe R, Urbach V. 2018. Specialized Pro-Resolving Lipid Mediators in Cystic Fibrosis. Int J Mol Sci 19: 2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferri G, Serano M, Isopi E, Mucci M, Mattoscio D, et al. 2023. Resolvin D1 improves airway inflammation and exercise capacity in cystic fibrosis lung disease. FASEB J. 37: e23233. [DOI] [PubMed] [Google Scholar]
- 103.Recchiuti A, Isopi E, Romano M, Mattoscio D. 2020. Roles of Specialized Pro-Resolving Lipid Mediators in Autophagy and Inflammation. Int J Mol Sci 21: 6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thornton JM, Walker JM, Sundarasivarao PYK, Spur BW, Rodriguez A, Yin K. 2021. Lipoxin A4 promotes reduction and antibiotic efficacy against Pseudomonas aeruginosa biofilm. Prostaglandins Other Lipid Mediat 152: 106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sham HP, Walker KH, Abdulnour RE, Krishnamoorthy N, Douda DN, et al. 2018. 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-kappaB Regulators in Bacterial Pneumonia. J Immunol 200: 2757–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tavares LP, Brüggemann TR, Rezende RM, Machado MG, Cagnina RE, et al. 2022. Cysteinyl Maresins Reprogram Macrophages to Protect Mice from Streptococcus pneumoniae after Influenza A Virus Infection. mBio 13: e0126722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walker KH, Krishnamoorthy N, Brüggemann TR, Shay AE, Serhan CN, Levy BD. 2021. Protectins PCTR1 and PD1 Reduce Viral Load and Lung Inflammation During Respiratory Syncytial Virus Infection in Mice. Front Immunol 12: 704427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krishnamoorthy N, Walker KH, Brüggemann TR, Tavares LP, Smith EW, et al. 2023. The Maresin 1-LGR6 axis decreases respiratory syncytial virus-induced lung inflammation. Proc Natl Acad Sci U S A 120: e2206480120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jundi B, Lee DH, Jeon H, Duvall MG, Nijmeh J, et al. 2021. Inflammation resolution circuits are uncoupled in acute sepsis and correlate with clinical severity. JCI Insight: doi: 10.1172/jci.insight.148866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. 2015. Asthma. Nat Rev Dis Primers 1: 15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brusselle GG, Koppelman GH. 2022. Biologic Therapies for Severe Asthma. N Engl J Med 386: 157–71 [DOI] [PubMed] [Google Scholar]
- 112.Peh HY, Brüggemann TR, Duvall MG, Nshimiyimana R, Nijmeh J, et al. 2024. Resolvin D2 regulates type 2 inflammatory responses and promotes resolution of mouse allergic inflammation. Allergy 79: 739–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brüggemann TR, Peh HY, Tavares LP, Nijmeh J, Shay AE, et al. 2023. Eosinophil Phenotypes Are Functionally Regulated by Resolvin D2 during Allergic Lung Inflammation. Am J Respir Cell Mol Biol 69: 666–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duvall MG, Barnig C, Cernadas M, Ricklefs I, Krishnamoorthy N, et al. 2017. Natural killer cell-mediated inflammation resolution is disabled in severe asthma. Sci Immunol 2: eaam5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, et al. 2017. ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight 2: e93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tejera P, Abdulnour RE, Zhu Z, Su L, Levy BD, Christiani DC. 2020. Plasma Levels of Proresolving and Prophlogistic Lipid Mediators: Association With Severity of Respiratory Failure and Mortality in Acute Respiratory Distress Syndrome. Crit Care Explor 2: e0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, et al. 2015. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol 194: 863–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. 2005. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol 174: 5033–39 [DOI] [PubMed] [Google Scholar]
- 119.Abdulnour RE, Gunderson T, Barkas I, Timmons JY, Barnig C, et al. 2018. Early Intravascular Events Are Associated with Development of Acute Respiratory Distress Syndrome. A Substudy of the LIPS-A Clinical Trial. Am J Respir Crit Care Med 197: 1575–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chiarella SE, Barnes PJ. 2023. Endogenous inhibitory mechanisms in asthma. J Allergy Clin Immunol Glob 2: 100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, et al. 2014. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci U S A 111: 16526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, et al. 2013. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol 6: 256–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, et al. 2010. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol 184: 836–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Serhan CN, Petasis NA. 2011. Resolvins and protectins in inflammation-resolution. Chem. Rev 111: 5922–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sasaki K, Urabe D, Arai H, Arita M, Inoue M. 2011. Total synthesis and bioactivities of two proposed structures of maresin. Chem. Asian J 6: 534–43 [DOI] [PubMed] [Google Scholar]
- 126.Rodriguez AR, Spur BW. 2012. Total synthesis of Resolvin D1, a potent anti-inflammatory lipid mediator. Tetrahedron Lett. 53: 6990–94 [Google Scholar]
- 127.Vidar Hansen T, Serhan CN. 2022. Protectins: Their biosynthesis, metabolism and structure-functions. Biochem Pharmacol 206: 115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Balas L, Risé P, Gandrath D, Rovati G, Bolego C, et al. 2019. Rapid Metabolization of Protectin D1 by β-Oxidation of Its Polar Head Chain. J Med Chem 62: 9961–75 [DOI] [PubMed] [Google Scholar]
- 129.Reinertsen AF, Libreros S, Nshimiyimana R, Serhan CN, Hansen TV. 2023. Metabolization of Resolvin E4 by omega-oxidation in human neutrophils: synthesis and biological evaluation of 20-hydroxy-Resolvin E4 (20-OH-RvE4). ACS Pharmacol. Transl. Sci doi: 10.1021/acsptsci.3c00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C-H, et al. 2010. Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors. Proc. Natl. Acad. Sci. U.S.A 107: 1660–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nunes VS, Abrahão O, Jr., Rogério AP, Serhan CN. 2023. ALX/FPR2 Activation by Stereoisomers of D1 Resolvins Elucidating with Molecular Dynamics Simulation. J Phys Chem B 127: 6479–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, et al. 2013. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A 110: 18232–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chiang N, Serhan CN. 2020. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 64: 443–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chiang N, de la Rosa X, Libreros S, Serhan CN. 2017. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J Immunol 198: 842–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, et al. 2013. Inhaled carbon monoxide accelerates resolution of inflammation via unique pro-resolving mediator--heme oxygenase-1 circuits. J. Immunol 190: 6378–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shinohara M, Kibi M, Riley IR, Chiang N, Dalli J, et al. 2014. Cell-cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1. Am J Physiol Lung Cell Mol Physiol 307: L746–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fredenburgh LE, Perrella MA, Barragan-Bradford D, Hess DR, Peters E, et al. 2018. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight 3: e124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Colby JK, Abdulnour RE, Sham HP, Dalli J, Colas RA, et al. 2016. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Protective for Injured Epithelia. Am J Pathol 186: 1801–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He P, Hao J, Kong LF, Wotan A, Yan P, et al. 2023. Resolvin and lipoxin metabolism network regulated by Hyssopus Cuspidatus Boriss extract in asthmatic mice. Prostaglandins Other Lipid Mediat: 106803. [DOI] [PubMed] [Google Scholar]
- 140.Johnson RK, Manke J, Campbell M, Armstrong M, Boorgula MP, et al. 2022. Lipid mediators are detectable in the nasal epithelium and differ by asthma status in female subjects. J Allergy Clin Immunol 150: 965–71.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Archambault AS, Zaid Y, Rakotoarivelo V, Turcotte C, Doré É, et al. 2021. High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients. FASEB J. 35: e21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.So J, Wu D, Lichtenstein AH, Tai AK, Matthan NR, et al. 2021. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis 316: 90–98 [DOI] [PubMed] [Google Scholar]
- 143.Barden A, Phillips M, Hill LM, Fletcher EM, Mas E, et al. 2018. Antiemetic doses of dexamethasone and their effects on immune cell populations and plasma mediators of inflammation resolution in healthy volunteers. Prostaglandins Leukot Essent Fatty Acids 139: 31–39 [DOI] [PubMed] [Google Scholar]
- 144.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. 2014. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med 211: 1037–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tracey KJ. 2002. The inflammatory reflex. Nature 420: 853–9 [DOI] [PubMed] [Google Scholar]
- 146.Huang Y, Dong S, Li X, Shi J, Zhang Y, et al. 2024. VNS-mediated α7nAChR signaling promotes SPM synthesis via regulation of netrin-1 expression during LPS-induced ALI. FASEB J. 38: e9664. [DOI] [PubMed] [Google Scholar]
- 147.Rao Z, Brunner E, Giszas B, Iyer-Bierhoff A, Gerstmeier J, et al. 2023. Glucocorticoids regulate lipid mediator networks by reciprocal modulation of 15-lipoxygenase isoforms affecting inflammation resolution. Proc Natl Acad Sci U S A 120: e2302070120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, et al. 2004. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med 350: 560–69 [DOI] [PubMed] [Google Scholar]
- 149.Funk CD. 2001. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 294: 1871–75 [DOI] [PubMed] [Google Scholar]
- 150.Arita M, Clish CB, Serhan CN. 2005. The contributions of aspirin and microbial oxygenase in the biosynthesis of anti-inflammatory resolvins: Novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem. Biophy. Res. Commun 338: 149–57 [DOI] [PubMed] [Google Scholar]
- 151.Capdevila JH, Falck JR, Dishman E, Karara A. 1990. Cytochrome P-450 arachidonate oxygenase. In Arachidonate Related Lipid Mediators, ed. Murphy RC, Fitzpatrick FA, pp. 385–94. San Diego: Academic Press; [DOI] [PubMed] [Google Scholar]
- 152.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, et al. 2011. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol 18: 976–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hamidzadeh K, Westcott J, Wourms N, Shay AE, Panigrahy A, et al. 2022. A newly synthesized 17-epi-NeuroProtectin D1/17-epi-Protectin D1: Authentication and functional regulation of Inflammation-Resolution. Biochem Pharmacol 203: 115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Malawista SE, de Boisfleury Chevance A, van Damme J, Serhan CN. 2008. Tonic inhibition of chemotaxis in human plasma. Proc Natl Acad Sci U S A 105: 17949–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hopke A, Lin T, Scherer AK, Shay AE, Timmer KD, et al. 2022. Transcellular biosynthesis of leukotriene B(4) orchestrates neutrophil swarming to fungi. iScience 25: 105226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dalli J, Chiang N, Serhan CN. 2014. Identification of sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. U.S.A 111: E4753–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dalli J, Vlasakov I, Riley IR, Rodriguez AR, Spur B, et al. 2016. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA 113: 12232–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, et al. 2006. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol 176: 1848–59 [DOI] [PubMed] [Google Scholar]
- 159.Ramon S, Dalli J, Sanger JM, Winkler JW, Aursnes M, et al. 2016. The Protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation Am. J. Pathol 186: 962–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Townsend EA, Guadarrama A, Shi L, Roti Roti E, Denlinger LC. 2023. P2X(7) signaling influences the production of pro-resolving and pro-inflammatory lipid mediators in alveolar macrophages derived from individuals with asthma. Am J Physiol Lung Cell Mol Physiol 325: L399–L410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Puzzovio PG, Pahima H, George T, Mankuta D, Eliashar R, et al. 2023. Mast cells contribute to the resolution of allergic inflammation by releasing resolvin D1. Pharmacol Res 189: 106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cox R Jr., Phillips O, Fukumoto J, Fukumoto I, Tamarapu Parthasarathy P, et al. 2015. Resolvins Decrease Oxidative Stress Mediated Macrophage and Epithelial Cell Interaction through Decreased Cytokine Secretion. PLoS One 10: e0136755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koltsida O, Karamnov S, Pyrillou K, Vickery T, Chairakaki AD, et al. 2013. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med 5: 762–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Navarini L, Vomero M, Currado D, Berardicurti O, Biaggi A, et al. 2023. The specialized pro-resolving lipid mediator Protectin D1 affects macrophages differentiation and activity in Adult-onset Still's disease and COVID-19, two hyperinflammatory diseases sharing similar transcriptomic profiles. Front Immunol 14: 1148268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Miyata J, Yokokura Y, Moro K, Arai H, Fukunaga K, Arita M. 2021. 12/15-Lipoxygenase Regulates IL-33-Induced Eosinophilic Airway Inflammation in Mice. Front Immunol 12: 687192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Serhan CN, Fahlstadius P, Dahlén SE, Hamberg M, Samuelsson B. 1985. Biosynthesis and biological activities of lipoxins. Advances in Prostaglandin, Thromboxane, & Leukotriene Research 15: 163–6 [PubMed] [Google Scholar]


