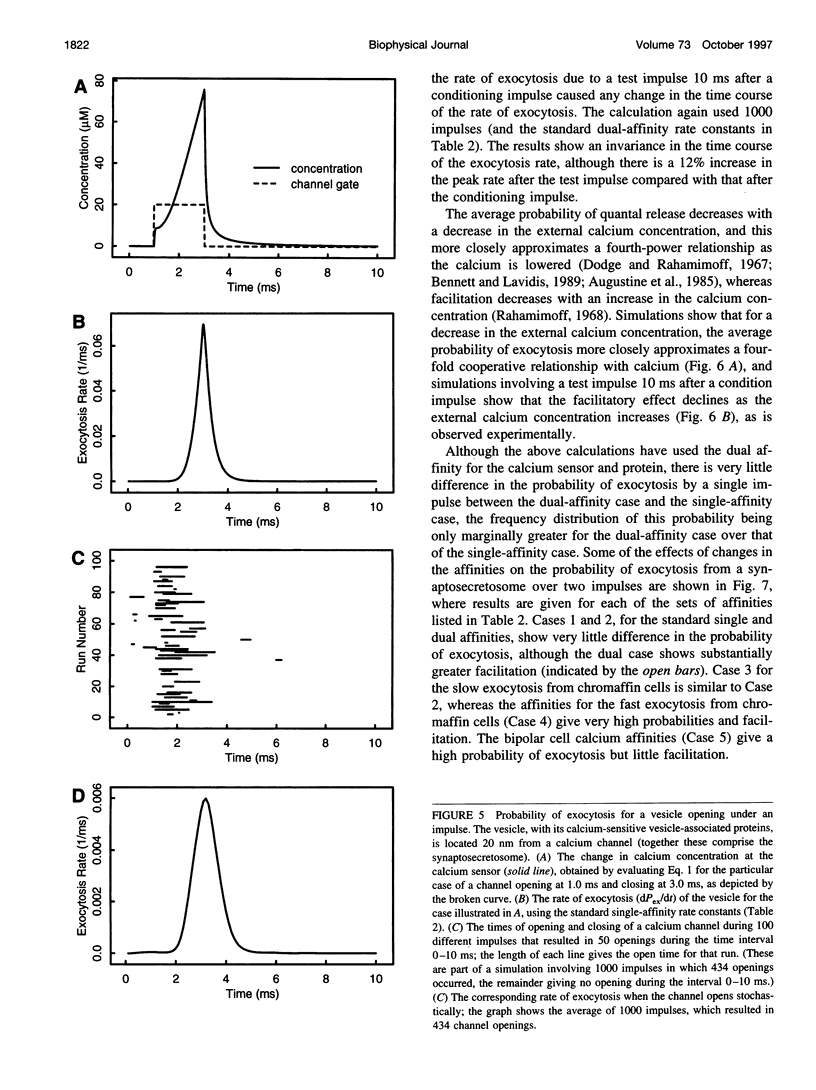
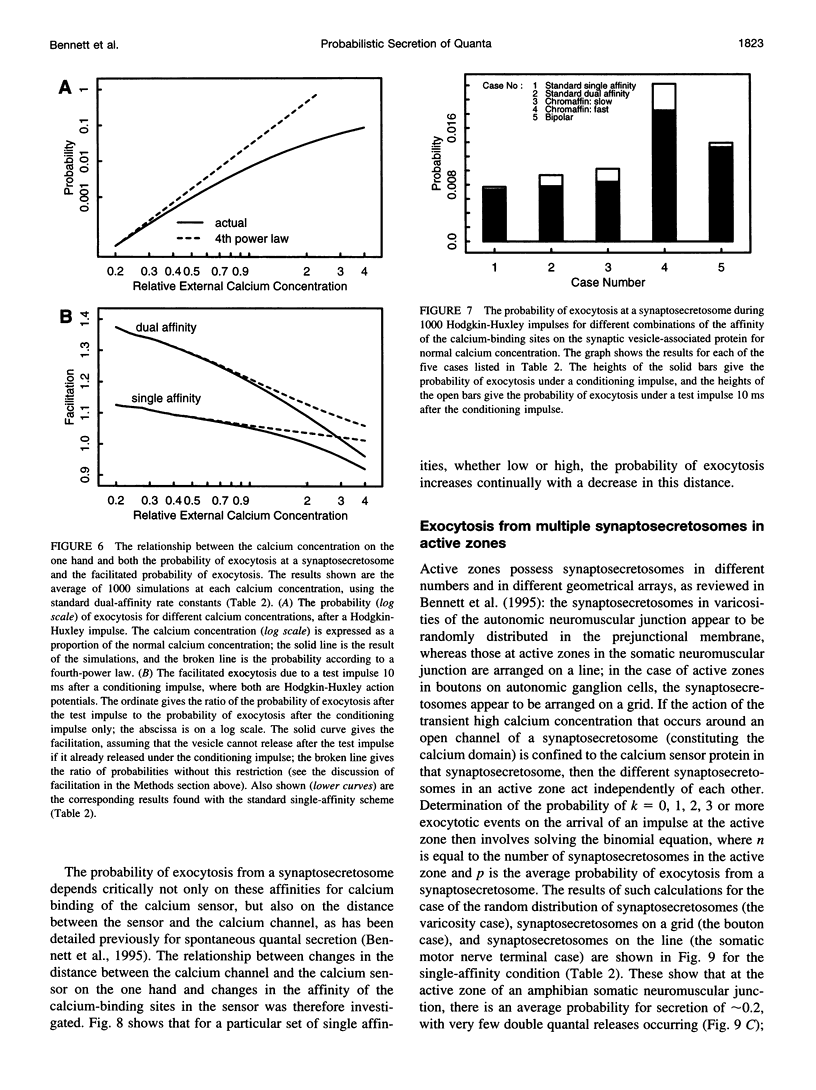
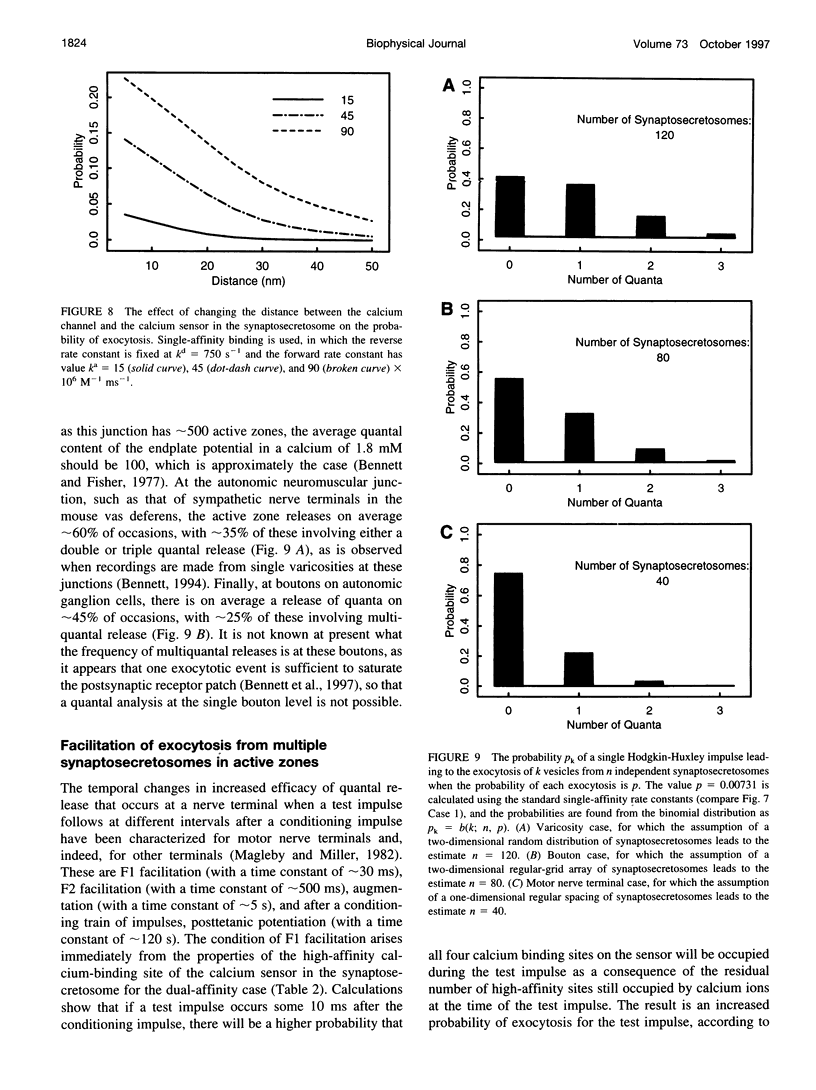
Abstract
A quantum of transmitter may be released upon the arrival of a nerve impulse if the influx of calcium ions through a nearby voltage-dependent calcium channel is sufficient to activate the vesicle-associated calcium sensor protein that triggers exocytosis. A synaptic vesicle, together with its calcium sensor protein, is often found complexed with the calcium channel in active zones to form what will be called a "synaptosecretosome." In the present work, a stochastic analysis is given of the conditions under which a quantum is released from the synaptosecretosome by a nerve impulse. The theoretical treatment considers the rise of calcium at the synaptosecretosome after the stochastic opening of a calcium channel at some time during the impulse, followed by the stochastic binding of calcium to the vesicle-associated protein and the probability of this leading to exocytosis. This allows determination of the probabilities that an impulse will release 0, 1, 2,... quanta from an active zone, whether this is in a varicosity, a bouton, or a motor endplate. A number of experimental observations of the release of transmitter at the active zones of sympathetic varicosities and boutons as well as somatic motor endplates are described by this analysis. These include the likelihood of the secretion of only one quantum at an active zone of endplates and of more than one quantum at an active zone of a sympathetic varicosity. The fourth-power relationship between the probability of transmitter release at the active zones of sympathetic varicosities and motor endplates and the external calcium concentration is also explained by this approach. So, too, is the fact that the time course of the increased rate of quantal secretion from a somatic active zone after an impulse is invariant with changes in the amount of calcium that enters through its calcium channel, whether due to changes consequent on the actions of autoreceptor agents such as adenosine or to facilitation. The increased probability of quantal release that occurs during F1 facilitation at the active zones of motor endplates and sympathetic boutons is predicted by the residual binding of calcium to a high-affinity site on the vesicle-associated protein. The concept of the stochastic operation of a synaptosecretosome can accommodate most phenomena involving the release of transmitter quanta at these synapses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharon S., Parnas H., Parnas I. The magnitude and significance of Ca2+ domains for release of neurotransmitter. Bull Math Biol. 1994 Nov;56(6):1095–1119. doi: 10.1007/BF02460288. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Farnell L., Gibson W. G., Lavidis N. A. Synaptic transmission at visualized sympathetic boutons: stochastic interaction between acetylcholine and its receptors. Biophys J. 1997 Apr;72(4):1595–1606. doi: 10.1016/S0006-3495(97)78806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Fisher C. The effects of calcium ions on the binomial parameters that control acetylcholine release during trains of nerve impulses at amphibian neuromuscular synapses. J Physiol. 1977 Oct;271(3):673–698. doi: 10.1113/jphysiol.1977.sp012020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Gibson W. G., Robinson J. Probabilistic secretion of quanta: spontaneous release at active zones of varicosities, boutons, and endplates. Biophys J. 1995 Jul;69(1):42–56. doi: 10.1016/S0006-3495(95)79873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Ho S. Probabilistic secretion of quanta from nerve terminals in avian ciliary ganglia modulated by adenosine. J Physiol. 1991;440:513–527. doi: 10.1113/jphysiol.1991.sp018722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Jones P., Lavidis N. A. The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular synapses. J Physiol. 1986 Oct;379:257–274. doi: 10.1113/jphysiol.1986.sp016252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Karunanithi S., Lavidis N. A. Probabilistic secretion of quanta from nerve terminals in toad (Bufo marinus) muscle modulated by adenosine. J Physiol. 1991 Feb;433:421–434. doi: 10.1113/jphysiol.1991.sp018435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A., Lavidis-Armson F. The probability of quantal secretion at release sites of different length in toad (Bufo marinus) muscle. J Physiol. 1989 Nov;418:235–249. doi: 10.1113/jphysiol.1989.sp017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Quantal secretion from single visualized synaptic varicosities of sympathetic nerve terminals. Adv Second Messenger Phosphoprotein Res. 1994;29:399–423. doi: 10.1016/s1040-7952(06)80028-5. [DOI] [PubMed] [Google Scholar]
- Bertram R., Sherman A., Stanley E. F. Single-domain/bound calcium hypothesis of transmitter release and facilitation. J Neurophysiol. 1996 May;75(5):1919–1931. doi: 10.1152/jn.1996.75.5.1919. [DOI] [PubMed] [Google Scholar]
- Blundon J. A., Wright S. N., Brodwick M. S., Bittner G. D. Presynaptic calcium-activated potassium channels and calcium channels at a crayfish neuromuscular junction. J Neurophysiol. 1995 Jan;73(1):178–189. doi: 10.1152/jn.1995.73.1.178. [DOI] [PubMed] [Google Scholar]
- Borst J. G., Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996 Oct 3;383(6599):431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Brain K. L., Bennett M. R. Calcium in sympathetic varicosities of mouse vas deferens during facilitation, augmentation and autoinhibition. J Physiol. 1997 Aug 1;502(Pt 3):521–536. doi: 10.1111/j.1469-7793.1997.521bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain K. L., Bennett M. R. Calcium in the nerve terminals of chick ciliary ganglia during facilitation, augmentation and potentiation. J Physiol. 1995 Dec 15;489(Pt 3):637–648. doi: 10.1113/jphysiol.1995.sp021079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Calcium buffering in squid axons. Annu Rev Biophys Bioeng. 1978;7:363–392. doi: 10.1146/annurev.bb.07.060178.002051. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Clay J. R., DeFelice L. J. Relationship between membrane excitability and single channel open-close kinetics. Biophys J. 1983 May;42(2):151–157. doi: 10.1016/S0006-3495(83)84381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Tank D. W. A quantitative measurement of the dependence of short-term synaptic enhancement on presynaptic residual calcium. J Neurosci. 1994 Oct;14(10):5885–5902. doi: 10.1523/JNEUROSCI.14-10-05885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour A. H., Lipscombe D., Tsien R. W. Multiple modes of N-type calcium channel activity distinguished by differences in gating kinetics. J Neurosci. 1993 Jan;13(1):181–194. doi: 10.1523/JNEUROSCI.13-01-00181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon P. G., Henderson E., Stanley E. F. Localization of individual calcium channels at the release face of a presynaptic nerve terminal. Neuron. 1994 Dec;13(6):1275–1280. doi: 10.1016/0896-6273(94)90414-6. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. I. Activation kinetics and pharmacology. J Gen Physiol. 1989 Jul;94(1):151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H., Zucker R. S. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994 Oct 13;371(6498):603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Katz E., Ferro P. A., Cherksey B. D., Sugimori M., Llinás R., Uchitel O. D. Effects of Ca2+ channel blockers on transmitter release and presynaptic currents at the frog neuromuscular junction. J Physiol. 1995 Aug 1;486(Pt 3):695–706. doi: 10.1113/jphysiol.1995.sp020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavidis N. A., Bennett M. R. Probabilistic secretion of quanta from successive sets of visualized varicosities along single sympathetic nerve terminals. J Auton Nerv Syst. 1993 Apr;43(1):41–50. doi: 10.1016/0165-1838(93)90320-t. [DOI] [PubMed] [Google Scholar]
- Li C., Davletov B. A., Südhof T. C. Distinct Ca2+ and Sr2+ binding properties of synaptotagmins. Definition of candidate Ca2+ sensors for the fast and slow components of neurotransmitter release. J Biol Chem. 1995 Oct 20;270(42):24898–24902. doi: 10.1074/jbc.270.42.24898. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Time resolved calcium microdomains and synaptic transmission. J Physiol Paris. 1995;89(2):77–81. doi: 10.1016/0928-4257(96)80554-7. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod G. T., Lavidis N. A., Bennett M. R. Calcium dependence of quantal secretion from visualized sympathetic nerve varicosities on the mouse vas deferens. J Physiol. 1994 Oct 1;480(Pt 1):61–70. doi: 10.1113/jphysiol.1994.sp020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. Facilitation, augmentation, and potentiation of transmitter release. Prog Brain Res. 1979;49:175–182. doi: 10.1016/S0079-6123(08)64631-2. [DOI] [PubMed] [Google Scholar]
- Martin-Moutot N., Charvin N., Leveque C., Sato K., Nishiki T., Kozaki S., Takahashi M., Seagar M. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. J Biol Chem. 1996 Mar 22;271(12):6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- O'Connor V. M., Shamotienko O., Grishin E., Betz H. On the structure of the 'synaptosecretosome'. Evidence for a neurexin/synaptotagmin/syntaxin/Ca2+ channel complex. FEBS Lett. 1993 Jul 12;326(1-3):255–260. doi: 10.1016/0014-5793(93)81802-7. [DOI] [PubMed] [Google Scholar]
- Parnas H., Hovav G., Parnas I. Effect of Ca2+ diffusion on the time course of neurotransmitter release. Biophys J. 1989 May;55(5):859–874. doi: 10.1016/S0006-3495(89)82885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Delaney K. R., Tank D. W. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994 Feb;14(2):523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Charlton M. P. Frequency facilitation is not caused by residual ionized calcium at the frog neuromuscular junction. Ann N Y Acad Sci. 1991;635:492–494. doi: 10.1111/j.1749-6632.1991.tb36537.x. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993 Dec;11(6):1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Hans M., Zipser K., Augustine G. J. Role of residual calcium in synaptic depression and posttetanic potentiation: fast and slow calcium signaling in nerve terminals. Neuron. 1991 Dec;7(6):915–926. doi: 10.1016/0896-6273(91)90337-y. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995 Jun 22;375(6533):645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Tanabe N., Kijima H. Ca(2+)-dependent and -independent components of transmitter release at the frog neuromuscular junction. J Physiol. 1992 Sep;455:271–289. doi: 10.1113/jphysiol.1992.sp019301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank D. W., Regehr W. G., Delaney K. R. A quantitative analysis of presynaptic calcium dynamics that contribute to short-term enhancement. J Neurosci. 1995 Dec;15(12):7940–7952. doi: 10.1523/JNEUROSCI.15-12-07940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson P. C., Lavidis N. A., Robinson J., Bennett M. R. Probabilistic secretion of quanta at somatic motor-nerve terminals: the fusion-pore model, quantal detection and autoinhibition. Philos Trans R Soc Lond B Biol Sci. 1995 Aug 29;349(1328):197–214. doi: 10.1098/rstb.1995.0103. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Estimating the timing of quantal releases during end-plate currents at the frog neuromuscular junction. J Physiol. 1988 Aug;402:595–603. doi: 10.1113/jphysiol.1988.sp017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow J. L., Duffy S. N., Charlton M. P. Homosynaptic facilitation of transmitter release in crayfish is not affected by mobile calcium chelators: implications for the residual ionized calcium hypothesis from electrophysiological and computational analyses. J Neurophysiol. 1994 Oct;72(4):1769–1793. doi: 10.1152/jn.1994.72.4.1769. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Angus J. A. Effects of N-, P- and Q-type neuronal calcium channel antagonists on mammalian peripheral neurotransmission. Br J Pharmacol. 1996 Sep;119(1):49–56. doi: 10.1111/j.1476-5381.1996.tb15676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994 Feb;14(2):645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada W. M., Zucker R. S. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992 Mar;61(3):671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Preferential inhibition of omega-conotoxin-sensitive presynaptic Ca2+ channels by adenosine autoreceptors. Nature. 1993 Sep 16;365(6443):256–258. doi: 10.1038/365256a0. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Oho C., Omori A., Kuwahara R., Ito T., Takahashi M. HPC-1 is associated with synaptotagmin and omega-conotoxin receptor. J Biol Chem. 1992 Dec 15;267(35):24925–24928. [PubMed] [Google Scholar]
- Yoshikami D., Bagabaldo Z., Olivera B. M. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]
- Zefirov A., Benish T., Fatkullin N., Cheranov S., Khazipov R. Localization of active zones. Nature. 1995 Aug 3;376(6539):393–394. doi: 10.1038/376393b0. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Far O., Charvin N., Leveque C., Martin-Moutot N., Takahashi M., Seagar M. J. Interaction of a synaptobrevin (VAMP)-syntaxin complex with presynaptic calcium channels. FEBS Lett. 1995 Mar 13;361(1):101–105. doi: 10.1016/0014-5793(95)00156-4. [DOI] [PubMed] [Google Scholar]


