Abstract
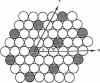
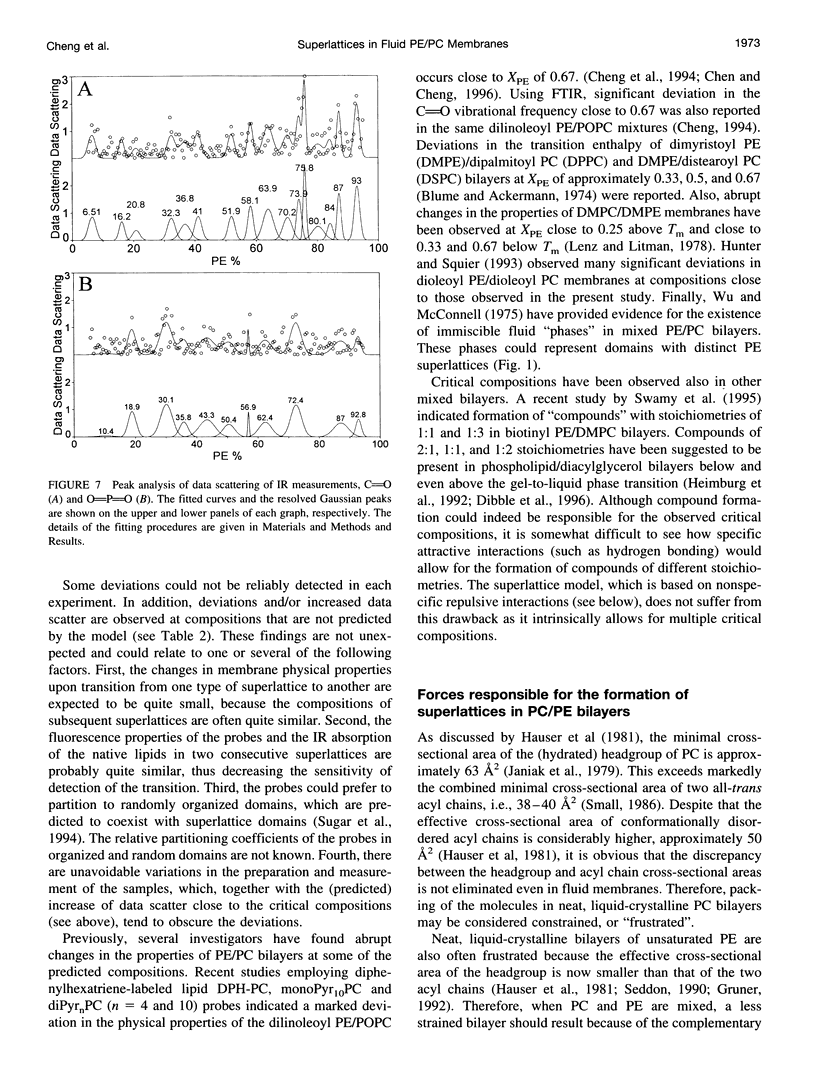
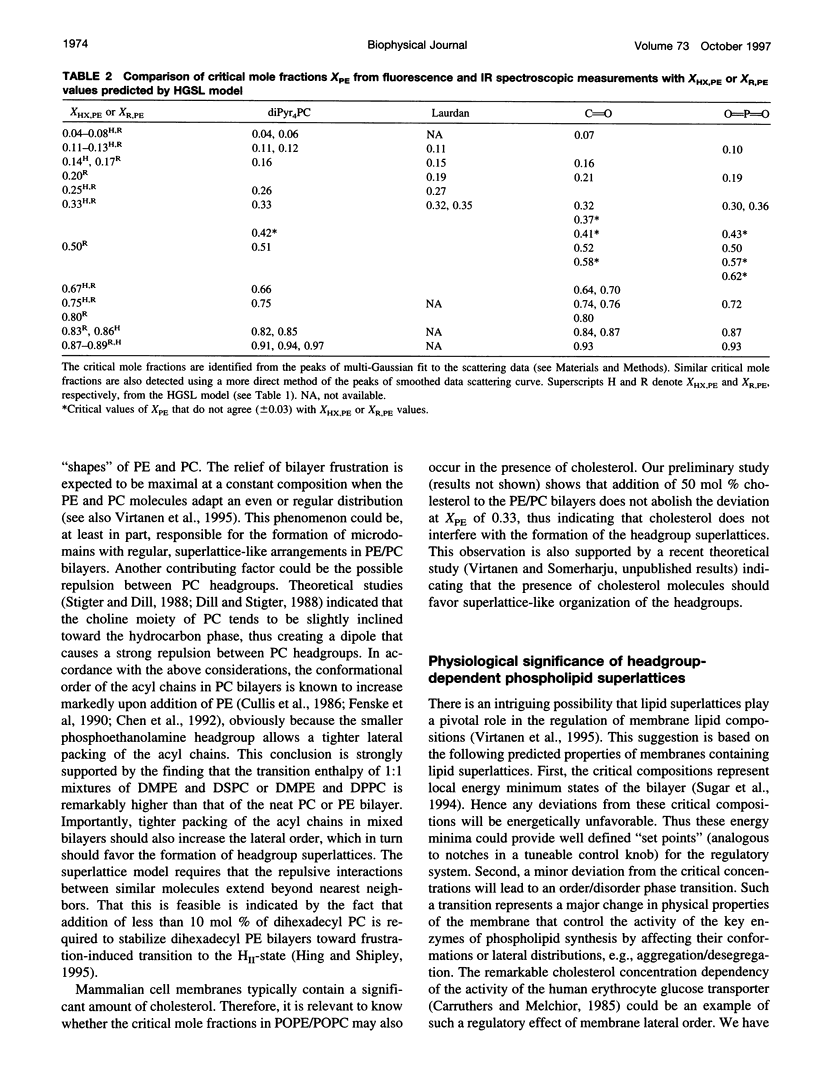
Recently, evidence for cholesterol and phosphatidylcholine (PC) molecules to adapt superlattice arrangements in fluid lipid bilayers has been presented. Whether superlattice arrangements exist in other biologically relevant lipid membranes, such as phosphatidylethanolamine (PE)/PC, is still speculative. In this study, we have examined the physical properties of fluid 1-palmitoyl-2-oleoyl-PC (POPC) and 1-palmitoyl-2-oleoyl-PE (POPE) binary mixtures as a function of the POPE mole fraction (X(PE)) using fluorescence and Fourier transform infrared spectroscopy. At 30 degrees C, i.e., above the Tm of POPE and POPC, deviations, or dips, as well as local data scattering in the excimer-to-monomer fluorescence intensity ratio of intramolecular excimer forming dipyrenylphosphatidylcholine probe in POPE/POPC mixtures were detected at X(PE) approximately 0.04, 0.11, 0.16, 0.26, 0.33, 0.51, 0.66, 0.75, 0.82, 0.91, and 0.94. The above critical values of X(PE) coincide (within +/-0.03) with the critical mole fractions X(HX,PE) or X(R,PE) predicted by a headgroup superlattice model, which assumes that the lipid headgroups form hexagonal or rectangular superlattice, respectively, in the bilayer. Other spectroscopic data, generalized polarization of Laurdan and infrared carbonyl and phosphate stretching frequency, were also collected. Similar agreements between some of the observed critical values of X(PE) from these data and the X(HX,PE) or X(R,PE) values were also found. However, all techniques yielded critical values of X(PE) (e.g., 0.42 and 0.58) that cannot be explained by the present headgroup superlattice model. The effective cross-sectional area of the PE headgroup is smaller than that of the acyl chains. Hence, the relief of "packing frustration" of PE in the presence of PC (larger headgroup than PE) may be one of the major mechanisms in driving the PE and PC components to superlattice-like lateral distributions in the bilayer. We propose that headgroup superlattices may play a significant role in the regulation of membrane lipid compositions in cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe G. B., Steim J. M. Membrane transitions in Gram-positive bacteria. Biochim Biophys Acta. 1971 Jun 1;233(3):810–814. doi: 10.1016/0005-2736(71)90182-9. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Suurkuusk J., Mountcastle D., Thompson T. E., Biltonen R. L. A calorimetric study of the thermotropic behavior of aqueous dispersions of natural and synthetic sphingomyelins. Biochemistry. 1976 Jun 1;15(11):2441–2447. doi: 10.1021/bi00656a030. [DOI] [PubMed] [Google Scholar]
- Berclaz T., McConnell H. M. Phase Equilibria in binary mixtures of dimyristoylphosphatidylcholine and cardiolipin. Biochemistry. 1981 Nov 10;20(23):6635–6640. doi: 10.1021/bi00526a018. [DOI] [PubMed] [Google Scholar]
- Blume A., Ackermann T. A calorimetric study of the lipid phase transitions in aqueous dispersions of phosphorylcholine--phosphorylethanolamine mixtures. FEBS Lett. 1974 Jul 1;43(1):71–74. doi: 10.1016/0014-5793(74)81108-7. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Munro S. Cholesterol and the Golgi apparatus. Science. 1993 Sep 3;261(5126):1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Seelig J. Influence of cholesterol on the polar region of phosphatidylcholine and phosphatidylethanolamine bilayers. Biochemistry. 1978 Jan 24;17(2):381–384. doi: 10.1021/bi00595a029. [DOI] [PubMed] [Google Scholar]
- Brown P. M., Steers J., Hui S. W., Yeagle P. L., Silvius J. R. Role of head group structure in the phase behavior of amino phospholipids. 2. Lamellar and nonlamellar phases of unsaturated phosphatidylethanolamine analogues. Biochemistry. 1986 Jul 29;25(15):4259–4267. doi: 10.1021/bi00363a013. [DOI] [PubMed] [Google Scholar]
- Carruthers A., Melchior D. L. Transport of alpha- and beta-D-glucose by the intact human red cell. Biochemistry. 1985 Jul 16;24(15):4244–4250. doi: 10.1021/bi00336a065. [DOI] [PubMed] [Google Scholar]
- Chen S. Y., Cheng K. H. Detection of membrane packing defects by time-resolved fluorescence depolarization. Biophys J. 1996 Aug;71(2):878–884. doi: 10.1016/S0006-3495(96)79289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Cheng K. H., Van der Meer B. W. Quantitation of lateral stress in lipid layer containing nonbilayer phase preferring lipids by frequency-domain fluorescence spectroscopy. Biochemistry. 1992 Apr 21;31(15):3759–3768. doi: 10.1021/bi00130a005. [DOI] [PubMed] [Google Scholar]
- Cheng K. H. Infrared study of the bilayer stability behavior of binary and ternary phospholipid mixtures containing unsaturated phosphatidylethanolamine. Chem Phys Lipids. 1994 Mar 31;70(1):43–51. doi: 10.1016/0009-3084(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Lepock J. R., Hui S. W., Yeagle P. L. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. J Biol Chem. 1986 Apr 15;261(11):5081–5087. [PubMed] [Google Scholar]
- Cheng K. H., Ruymgaart L., Liu L. I., Somerharju P., Sugar I. P. Intramolecular excimer kinetics of fluorescent dipyrenyl lipids: 2. DOPE/DOPC membranes. Biophys J. 1994 Aug;67(2):914–921. doi: 10.1016/S0006-3495(94)80553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. H., Somerharju P., Sugar I. Detection and characterization of the onset of bilayer packing defects by nanosecond-resolved intramolecular excimer fluorescence spectroscopy. Chem Phys Lipids. 1994 Oct 20;74(1):49–64. doi: 10.1016/0009-3084(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Chong P. L. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10069–10073. doi: 10.1073/pnas.91.21.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):127–144. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Tsujita T., Brockman H. L. Enzymatic and physical characterization of diacylglycerol-phosphatidylcholine interactions in bilayers and monolayers. Biochemistry. 1989 Jan 10;28(1):32–40. doi: 10.1021/bi00427a006. [DOI] [PubMed] [Google Scholar]
- Dibble A. R., Hinderliter A. K., Sando J. J., Biltonen R. L. Lipid lateral heterogeneity in phosphatidylcholine/phosphatidylserine/diacylglycerol vesicles and its influence on protein kinase C activation. Biophys J. 1996 Oct;71(4):1877–1890. doi: 10.1016/S0006-3495(96)79387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill K. A., Stigter D. Lateral interactions among phosphatidylcholine and phosphatidylethanolamine head groups in phospholipid monolayers and bilayers. Biochemistry. 1988 May 3;27(9):3446–3453. doi: 10.1021/bi00409a048. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Virtanen J. A., Kinnunen P. K., Kasurinen J., Somerharju P. J. Conformation of phosphatidylcholine in neat and cholesterol-containing liquid-crystalline bilayers. Application of a novel method. Biochemistry. 1992 Sep 15;31(36):8560–8565. doi: 10.1021/bi00151a025. [DOI] [PubMed] [Google Scholar]
- Fenske D. B., Jarrell H. C., Guo Y., Hui S. W. Effect of unsaturated phosphatidylethanolamine on the chain order profile of bilayers at the onset of the hexagonal phase transition. A 2H NMR study. Biochemistry. 1990 Dec 25;29(51):11222–11229. doi: 10.1021/bi00503a010. [DOI] [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J. Plasma membrane caveolae mediate the efflux of cellular free cholesterol. Biochemistry. 1995 Nov 7;34(44):14288–14292. doi: 10.1021/bi00044a004. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Heimburg T., Würz U., Marsh D. Binary phase diagram of hydrated dimyristoylglycerol-dimyristoylphosphatidylcholine mixtures. Biophys J. 1992 Nov;63(5):1369–1378. doi: 10.1016/S0006-3495(92)81714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing F. S., Shipley G. G. Molecular interactions of ether-linked phospholipids. Biochemistry. 1995 Sep 19;34(37):11904–11909. doi: 10.1021/bi00037a031. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-wei S., McConnell H. Phase separations in phospholipd membranes. Biochemistry. 1975 Feb 25;14(4):847–854. doi: 10.1021/bi00675a032. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Lentz B. R., Litman B. J. Effect of head group on phospholipid mixing in small, unilamellar vesicles: mixtures of dimyristoylphosphatidylcholine and dimyristoylphosphatidylethanolamine. Biochemistry. 1978 Dec 12;17(25):5537–5543. doi: 10.1021/bi00618a032. [DOI] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Ravagnan G., Rusch R. M., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991 Jul;60(1):179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Giusti A. M., Raimondi M., Gratton E. Abrupt modifications of phospholipid bilayer properties at critical cholesterol concentrations. Biophys J. 1995 May;68(5):1895–1902. doi: 10.1016/S0006-3495(95)80367-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., van Loon D., Wirtz K. W. Determination of the acyl chain specificity of the bovine liver phosphatidylcholine transfer protein. Application of pyrene-labeled phosphatidylcholine species. Biochemistry. 1987 Nov 3;26(22):7193–7199. doi: 10.1021/bi00396a048. [DOI] [PubMed] [Google Scholar]
- Swamy M. J., Würz U., Marsh D. Phase polymorphism, molecular interactions, and miscibility of binary mixtures of dimyristoyl-N-biotinylphosphatidylethanolamine with dimyristoylphosphatidylcholine. Biochemistry. 1995 Jun 6;34(22):7295–7302. doi: 10.1021/bi00022a002. [DOI] [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Wieb van der Meer B., Chen S. Y. Evidence for a regular distribution of cholesterol in phospholipid bilayers from diphenylhexatriene fluorescence. Biophys J. 1995 May;68(5):1944–1951. doi: 10.1016/S0006-3495(95)80371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne J. F., Cézanne L., Lopez A., Piknova B., Schram V., Tournier J. F., Welby M. Lipid domains and lipid/protein interactions in biological membranes. Chem Phys Lipids. 1994 Sep 6;73(1-2):139–158. doi: 10.1016/0009-3084(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Virtanen J. A., Ruonala M., Vauhkonen M., Somerharju P. Lateral organization of liquid-crystalline cholesterol-dimyristoylphosphatidylcholine bilayers. Evidence for domains with hexagonal and centered rectangular cholesterol superlattices. Biochemistry. 1995 Sep 12;34(36):11568–11581. doi: 10.1021/bi00036a033. [DOI] [PubMed] [Google Scholar]
- Welti R., Glaser M. Lipid domains in model and biological membranes. Chem Phys Lipids. 1994 Sep 6;73(1-2):121–137. doi: 10.1016/0009-3084(94)90178-3. [DOI] [PubMed] [Google Scholar]