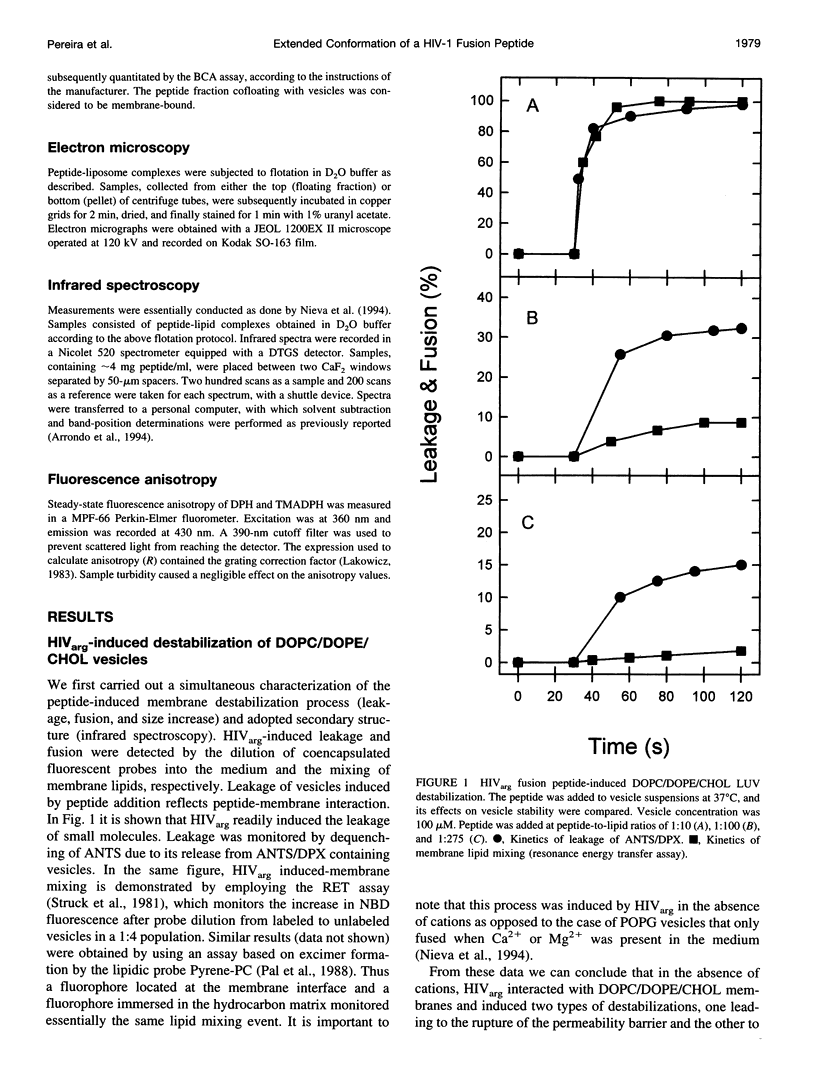
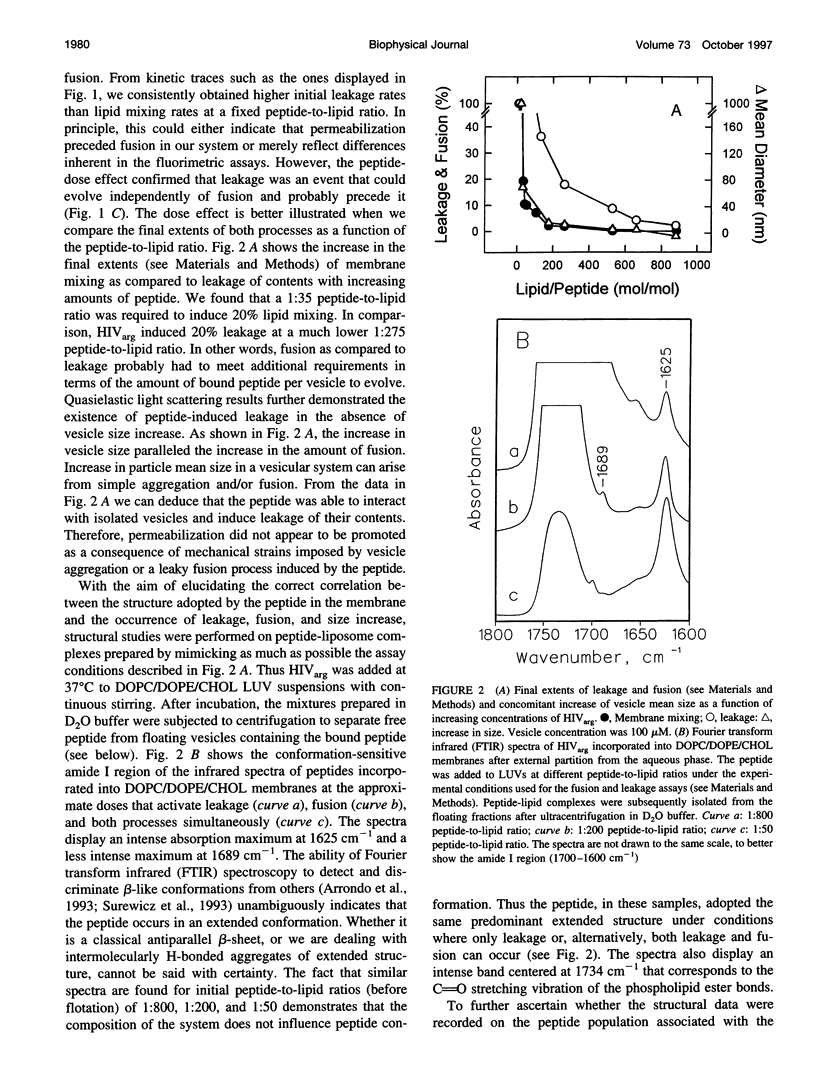
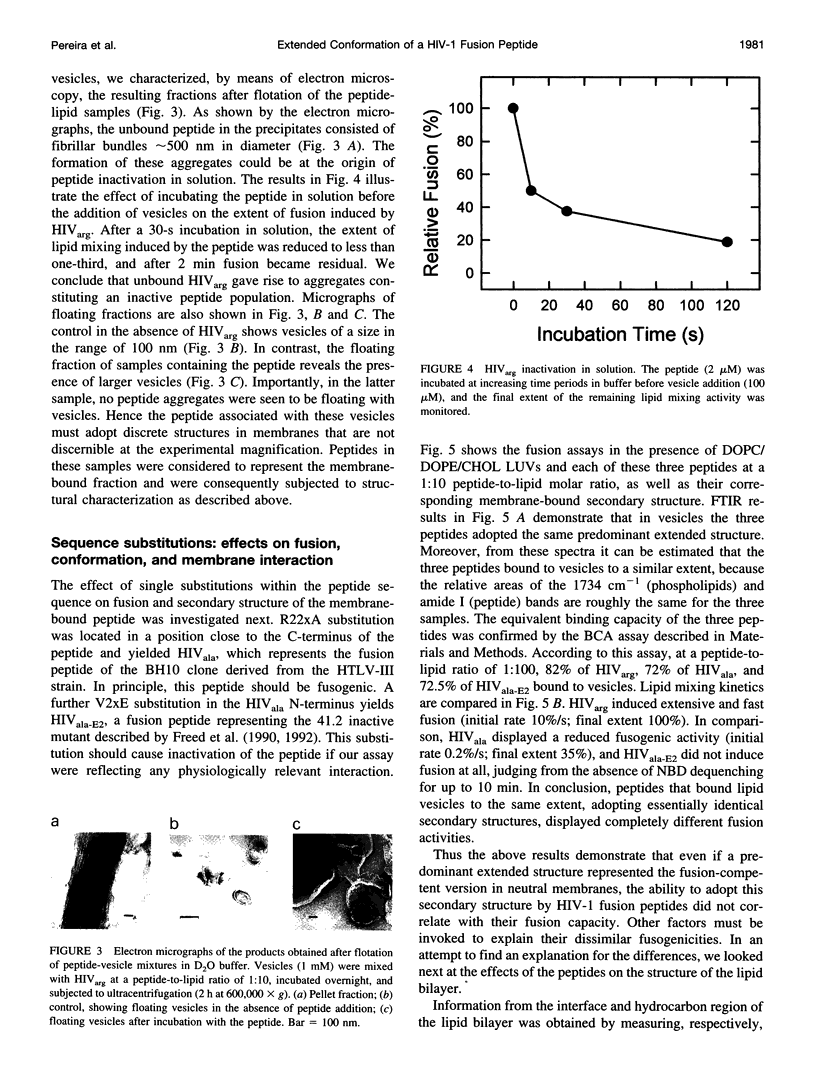
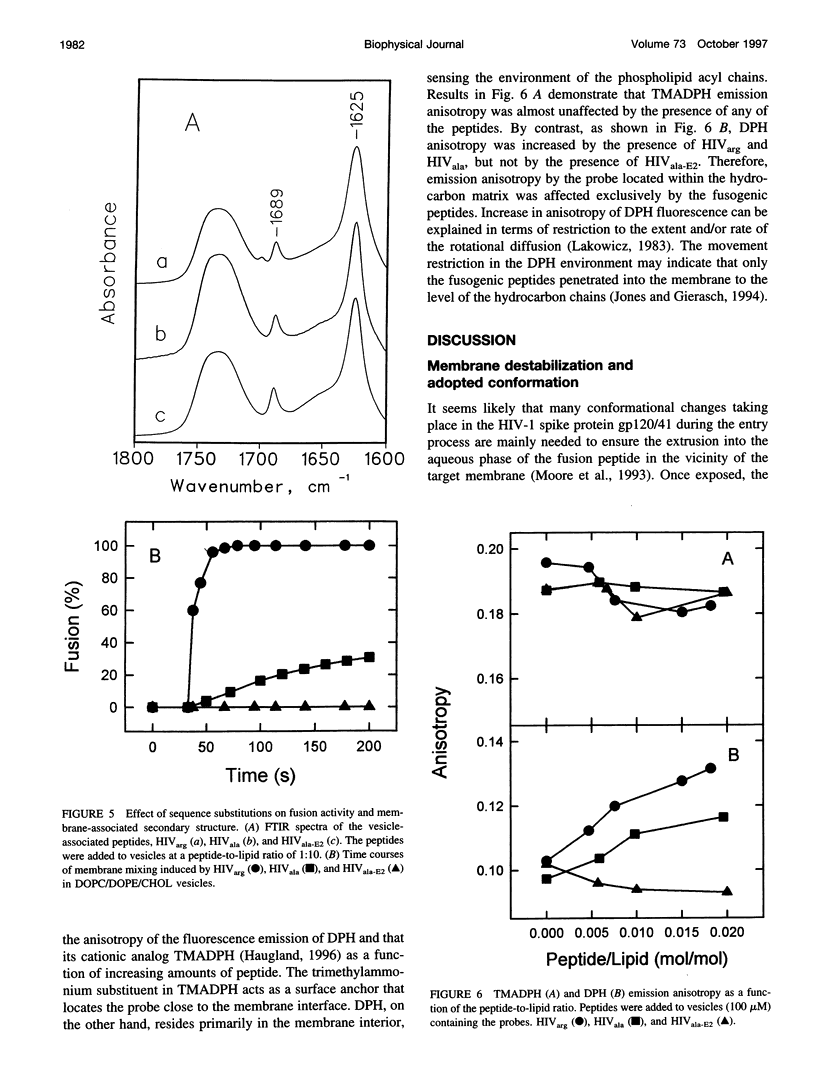
Abstract
The peptide HIV(arg), corresponding to a sequence of 23 amino acid residues at the N-terminus of HIV-1 gp41 (LAV1a strain), has the capacity to destabilize negatively charged large unilamellar vesicles. As revealed by infrared spectroscopy, the peptide associated with those vesicles showed conformational polymorphism: in the absence of cations the main structure was a pore-forming alpha-helix, whereas in the presence of Ca2+ the conformation switched to a fusogenic, predominantly extended beta-type structure. Here we show that an extended structure can also be involved in electrically neutral vesicle destabilization induced by the HIV-1 fusion peptide when it binds the vesicle from the aqueous phase. In the absence of cations, neutral liposomes composed of phosphatidylcholine, phosphatidylethanolamine, and cholesterol (molar ratio 1:1:1) selected for an extended structure that became fusogenic in a dose-dependent fashion. At subfusogenic doses this structure caused the release of trapped 8-aminonaphtalene-1,3,6-trisulfonic acid sodium salt/p-xylenebis(pyridinium)bromide from liposomes, indicating the existence of a peptide-mediated membrane destabilizing process before and independent of the development of fusion. When compared to HIV(arg), the fusion activity of HIV(ala) (bearing the R22 --> A substitution) was reduced by 70%. Fusogenicity was completely abolished when a second substitution (V2 --> E) was included to generate HIV(ala-E2), a sequence representing the N-terminus of an inactive gp41. However, the three sequences associated with vesicles to the same extent, and the three adopted a similar extended structure in the membrane. Whereas 1-(4-trimethylaminophenyl)-6-phenyl-1,3,5-hexatriene emission anisotropy was unaffected by the three peptides, DPH emission anisotropy in membranes was increased only by the fusogenic sequences. Taken together, our observations strongly argue that it is not an alpha-helical but an extended structure adopted by the HIV-1 fusion peptide what actively destabilizes cholesterol-containing, electrically neutral membranes. Moreover, membrane destabilization is modulated by the amino acid sequence in the extended structure. The effect displayed by the aforementioned V2 --> E substitution suggests that the fusion process described here could be reflecting a physiologically relevant phenomenon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloia R. C., Jensen F. C., Curtain C. C., Mobley P. W., Gordon L. M. Lipid composition and fluidity of the human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Feb;85(3):900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia R. C., Tian H., Jensen F. C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrondo J. L., Castresana J., Valpuesta J. M., Goñi F. M. Structure and thermal denaturation of crystalline and noncrystalline cytochrome oxidase as studied by infrared spectroscopy. Biochemistry. 1994 Sep 27;33(38):11650–11655. doi: 10.1021/bi00204a029. [DOI] [PubMed] [Google Scholar]
- Arrondo J. L., Muga A., Castresana J., Goñi F. M. Quantitative studies of the structure of proteins in solution by Fourier-transform infrared spectroscopy. Prog Biophys Mol Biol. 1993;59(1):23–56. doi: 10.1016/0079-6107(93)90006-6. [DOI] [PubMed] [Google Scholar]
- Bañuelos S., Muga A. Binding of molten globule-like conformations to lipid bilayers. Structure of native and partially folded alpha-lactalbumin bound to model membranes. J Biol Chem. 1995 Dec 15;270(50):29910–29915. doi: 10.1074/jbc.270.50.29910. [DOI] [PubMed] [Google Scholar]
- Bañuelos S., Muga A. Interaction of native and partially folded conformations of alpha-lactalbumin with lipid bilayers: characterization of two membrane-bound states. FEBS Lett. 1996 May 13;386(1):21–25. doi: 10.1016/0014-5793(96)00406-1. [DOI] [PubMed] [Google Scholar]
- Bosch M. L., Earl P. L., Fargnoli K., Picciafuoco S., Giombini F., Wong-Staal F., Franchini G. Identification of the fusion peptide of primate immunodeficiency viruses. Science. 1989 May 12;244(4905):694–697. doi: 10.1126/science.2541505. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Goto N. K. Folding proteins into membranes. Nat Struct Biol. 1996 Oct;3(10):815–818. doi: 10.1038/nsb1096-815. [DOI] [PubMed] [Google Scholar]
- Dragic T., Litwin V., Allaway G. P., Martin S. R., Huang Y., Nagashima K. A., Cayanan C., Maddon P. J., Koup R. A., Moore J. P. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996 Jun 20;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. H+- and Ca2+-induced fusion and destabilization of liposomes. Biochemistry. 1985 Jun 18;24(13):3099–3106. doi: 10.1021/bi00334a005. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Cheetham J. J., Epand R. F., Yeagle P. L., Richardson C. D., Rockwell A., Degrado W. F. Peptide models for the membrane destabilizing actions of viral fusion proteins. Biopolymers. 1992 Apr;32(4):309–314. doi: 10.1002/bip.360320403. [DOI] [PubMed] [Google Scholar]
- Feng Y., Broder C. C., Kennedy P. E., Berger E. A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 May 10;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Delwart E. L., Buchschacher G. L., Jr, Panganiban A. T. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987 Jul 31;50(3):327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Segrest J. P., Hunter E. Are fusion peptides really "sided" insertional helices? Cell. 1992 Aug 21;70(4):531–532. doi: 10.1016/0092-8674(92)90423-a. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Curtain C. C., Zhong Y. C., Kirkpatrick A., Mobley P. W., Waring A. J. The amino-terminal peptide of HIV-1 glycoprotein 41 interacts with human erythrocyte membranes: peptide conformation, orientation and aggregation. Biochim Biophys Acta. 1992 Aug 25;1139(4):257–274. doi: 10.1016/0925-4439(92)90099-9. [DOI] [PubMed] [Google Scholar]
- Gray C., Tatulian S. A., Wharton S. A., Tamm L. K. Effect of the N-terminal glycine on the secondary structure, orientation, and interaction of the influenza hemagglutinin fusion peptide with lipid bilayers. Biophys J. 1996 May;70(5):2275–2286. doi: 10.1016/S0006-3495(96)79793-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter C., James P., Bächi T., Semenza G., Brunner J. Hydrophobic binding of the ectodomain of influenza hemagglutinin to membranes occurs through the "fusion peptide". J Biol Chem. 1989 Apr 15;264(11):6459–6464. [PubMed] [Google Scholar]
- Hoyt D. W., Gierasch L. M. A peptide corresponding to an export-defective mutant OmpA signal sequence with asparagine in the hydrophobic core is unable to insert into model membranes. J Biol Chem. 1991 Aug 5;266(22):14406–14412. [PubMed] [Google Scholar]
- Jones J. D., Gierasch L. M. Effect of charged residue substitutions on the membrane-interactive properties of signal sequences of the Escherichia coli LamB protein. Biophys J. 1994 Oct;67(4):1534–1545. doi: 10.1016/S0006-3495(94)80627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Iwata T., Oyagi H., Aoyagi H., Ohno M., Anzai K., Kirino Y., Sugihara G. Effect of salts on conformational change of basic amphipathic peptides from beta-structure to alpha-helix in the presence of phospholipid liposomes and their channel-forming ability. Biochim Biophys Acta. 1993 Sep 5;1151(1):76–82. doi: 10.1016/0005-2736(93)90073-9. [DOI] [PubMed] [Google Scholar]
- Lu M., Blacklow S. C., Kim P. S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995 Dec;2(12):1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- Lüneberg J., Martin I., Nüssler F., Ruysschaert J. M., Herrmann A. Structure and topology of the influenza virus fusion peptide in lipid bilayers. J Biol Chem. 1995 Nov 17;270(46):27606–27614. doi: 10.1074/jbc.270.46.27606. [DOI] [PubMed] [Google Scholar]
- Martin I., Defrise-Quertain F., Decroly E., Vandenbranden M., Brasseur R., Ruysschaert J. M. Orientation and structure of the NH2-terminal HIV-1 gp41 peptide in fused and aggregated liposomes. Biochim Biophys Acta. 1993 Jan 18;1145(1):124–133. doi: 10.1016/0005-2736(93)90389-h. [DOI] [PubMed] [Google Scholar]
- Muga A., Neugebauer W., Hirama T., Surewicz W. K. Membrane interaction and conformational properties of the putative fusion peptide of PH-30, a protein active in sperm-egg fusion. Biochemistry. 1994 Apr 19;33(15):4444–4448. doi: 10.1021/bi00181a002. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Nir S., Muga A., Goñi F. M., Wilschut J. Interaction of the HIV-1 fusion peptide with phospholipid vesicles: different structural requirements for fusion and leakage. Biochemistry. 1994 Mar 22;33(11):3201–3209. doi: 10.1021/bi00177a009. [DOI] [PubMed] [Google Scholar]
- Pal R., Barenholz Y., Wagner R. R. Pyrene phospholipid as a biological fluorescent probe for studying fusion of virus membrane with liposomes. Biochemistry. 1988 Jan 12;27(1):30–36. doi: 10.1021/bi00401a006. [DOI] [PubMed] [Google Scholar]
- Pereira F. B., Goñi F. M., Nieva J. L. Liposome destabilization induced by the HIV-1 fusion peptide effect of a single amino acid substitution. FEBS Lett. 1995 Apr 3;362(2):243–246. doi: 10.1016/0014-5793(95)00257-a. [DOI] [PubMed] [Google Scholar]
- Rafalski M., Lear J. D., DeGrado W. F. Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry. 1990 Aug 28;29(34):7917–7922. doi: 10.1021/bi00486a020. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Jones J. D. Mode of membrane interaction of wild-type and mutant signal peptides of the Escherichia coli outer membrane protein A. J Biol Chem. 1994 Sep 23;269(38):23477–23483. [PubMed] [Google Scholar]
- Slepushkin V. A., Andreev S. M., Sidorova M. V., Melikyan G. B., Grigoriev V. B., Chumakov V. M., Grinfeldt A. E., Manukyan R. A., Karamov E. V. Investigation of human immunodeficiency virus fusion peptides. Analysis of interrelations between their structure and function. AIDS Res Hum Retroviruses. 1992 Jan;8(1):9–18. doi: 10.1089/aid.1992.8.9. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Wharton S. A., Skehel J. J., Wiley D. C. Studies of the membrane fusion activities of fusion peptide mutants of influenza virus hemagglutinin. J Virol. 1995 Nov;69(11):6643–6651. doi: 10.1128/jvi.69.11.6643-6651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H., Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993 Jan 19;32(2):389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- Takahashi S. Conformation of membrane fusion-active 20-residue peptides with or without lipid bilayers. Implication of alpha-helix formation for membrane fusion. Biochemistry. 1990 Jul 3;29(26):6257–6264. doi: 10.1021/bi00478a021. [DOI] [PubMed] [Google Scholar]
- Tamm L. K. Membrane insertion and lateral mobility of synthetic amphiphilic signal peptides in lipid model membranes. Biochim Biophys Acta. 1991 Jul 22;1071(2):123–148. doi: 10.1016/0304-4157(91)90021-n. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Shih I. L., Lees A. M., Lees R. S. Surface-induced conformational switching in amphiphilic peptide segments of apolipoproteins B and E and model peptides. Int J Pept Protein Res. 1993 Jun;41(6):536–547. doi: 10.1111/j.1399-3011.1993.tb00475.x. [DOI] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- White S. H., Wimley W. C., Selsted M. E. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995 Aug;5(4):521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Wild C., Dubay J. W., Greenwell T., Baird T., Jr, Oas T. G., McDanal C., Hunter E., Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimley W. C., White S. H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Biol. 1996 Oct;3(10):842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Vogel S. S., Chernomordik L. V. Mechanisms of membrane fusion. Annu Rev Biophys Biomol Struct. 1993;22:433–466. doi: 10.1146/annurev.bb.22.060193.002245. [DOI] [PubMed] [Google Scholar]



