Abstract
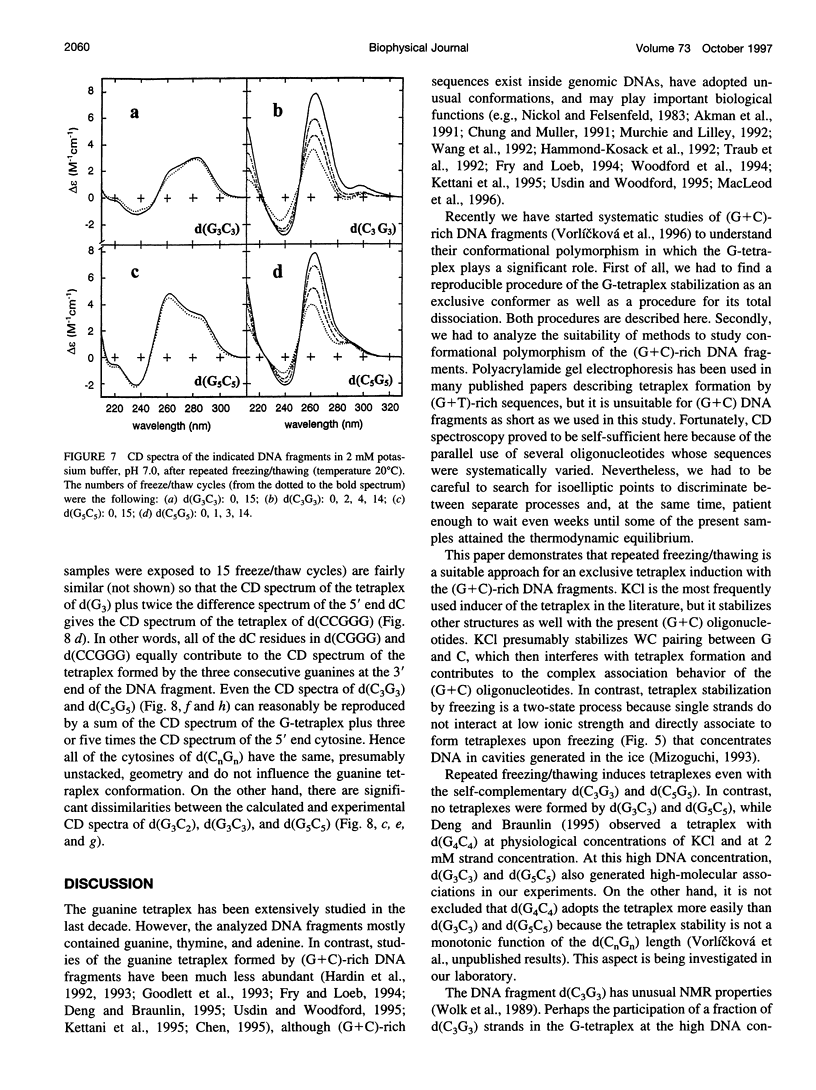
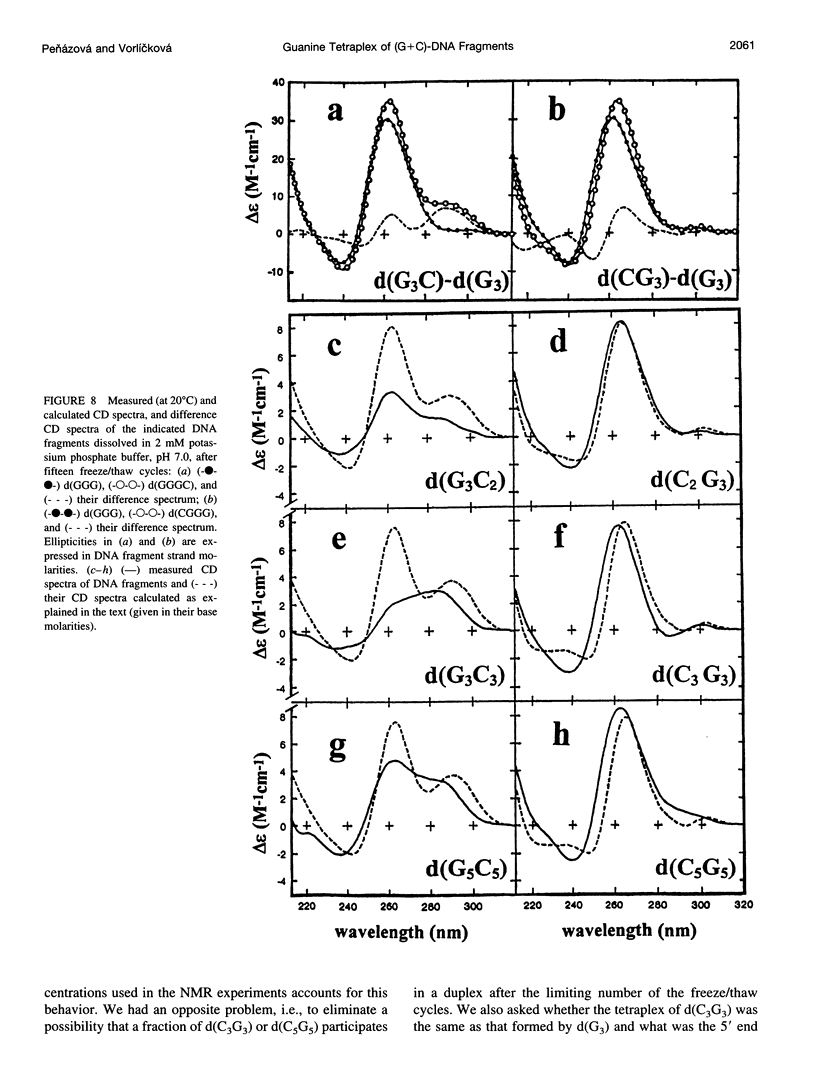
Using CD spectroscopy, guanine tetraplex formation was studied with short DNA fragments in which cytosine residues were systematically added to runs of guanine either at the 5' or 3' ends. Potassium cations induced the G-tetraplex more easily with fragments having the guanine run at the 5' end, which is just an opposite tendency to what was reported for (G+T) oligonucleotides. However, the present (G+C) fragments simultaneously adopted other conformers that complicated the analysis. We demonstrate that repeated freezing/thawing, performed at low ionic strength, is a suitable method to exclusively stabilize the tetraplex in the (G+C) DNA fragments. In contrast to KCl, the repeated freeze/thaw cycles better stabilized the tetraplex with fragments having the guanine run on the 3' end. The tendency of guanine blocks to generate the tetraplex destabilized the d(G5).d(C5) duplex whose strands dissociated, giving rise to a stable tetraplex of (dG5) and single-stranded (dC5). In contrast to d(G3C3) and d(G5C5), repeated freezing/thawing induced the tetraplex even with the self-complementary d(C3G3) or d(C5G5); hence the latter oligonucleotides preferred the tetraplex to the apparently very stable duplex. The tetraplexes only included guanine blocks while the 5' end cytosines interfered neither with the tetraplex formation nor the tetraplex structure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Murchie A. I., Lilley D. M. NMR study of parallel-stranded tetraplex formation by the hexadeoxynucleotide d(TG4T). Nature. 1992 Nov 19;360(6401):280–282. doi: 10.1038/360280a0. [DOI] [PubMed] [Google Scholar]
- Akman S. A., Lingeman R. G., Doroshow J. H., Smith S. S. Quadruplex DNA formation in a region of the tRNA gene supF associated with hydrogen peroxide mediated mutations. Biochemistry. 1991 Sep 3;30(35):8648–8653. doi: 10.1021/bi00099a022. [DOI] [PubMed] [Google Scholar]
- Balagurumoorthy P., Brahmachari S. K., Mohanty D., Bansal M., Sasisekharan V. Hairpin and parallel quartet structures for telomeric sequences. Nucleic Acids Res. 1992 Aug 11;20(15):4061–4067. doi: 10.1093/nar/20.15.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T. L., Fisher E. F., Ross S. L., Bready J. V., Qian Y. X., Bayewitch L. A., Cohen A. M., Herrera C. J., Hu S. S., Kramer T. B. The antiproliferative activity of c-myb and c-myc antisense oligonucleotides in smooth muscle cells is caused by a nonantisense mechanism. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):4051–4055. doi: 10.1073/pnas.92.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor C. R., Warshaw M. M., Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9(9):1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- Chen F. M. Acid-facilitated supramolecular assembly of G-quadruplexes in d(CGG)4. J Biol Chem. 1995 Sep 29;270(39):23090–23096. doi: 10.1074/jbc.270.39.23090. [DOI] [PubMed] [Google Scholar]
- Chen F. M. Intramolecular triplex formation of the purine.purine.pyrimidine type. Biochemistry. 1991 May 7;30(18):4472–4479. doi: 10.1021/bi00232a014. [DOI] [PubMed] [Google Scholar]
- Chen F. M. Sr2+ facilitates intermolecular G-quadruplex formation of telomeric sequences. Biochemistry. 1992 Apr 21;31(15):3769–3776. doi: 10.1021/bi00130a006. [DOI] [PubMed] [Google Scholar]
- Chung I. K., Muller M. T. Aggregates of oligo(dG) bind and inhibit topoisomerase II activity and induce formation of large networks. J Biol Chem. 1991 May 25;266(15):9508–9514. [PubMed] [Google Scholar]
- Deng H., Braunlin W. H. Duplex to quadruplex equilibrium of the self-complementary oligonucleotide d(GGGGCCCC). Biopolymers. 1995 Jun;35(6):677–681. doi: 10.1002/bip.360350613. [DOI] [PubMed] [Google Scholar]
- Fry M., Loeb L. A. The fragile X syndrome d(CGG)n nucleotide repeats form a stable tetrahelical structure. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4950–4954. doi: 10.1073/pnas.91.11.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELLERT M., LIPSETT M. N., DAVIES D. R. Helix formation by guanylic acid. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett D. R., Camp D. G., 2nd, Hardin C. C., Corregan M., Smith R. D. Direct observation of a DNA quadruplex by electrospray ionization mass spectrometry. Biol Mass Spectrom. 1993 Mar;22(3):181–183. doi: 10.1002/bms.1200220307. [DOI] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Guo Q., Lu M., Kallenbach N. R. Effect of thymine tract length on the structure and stability of model telomeric sequences. Biochemistry. 1993 Apr 13;32(14):3596–3603. doi: 10.1021/bi00065a010. [DOI] [PubMed] [Google Scholar]
- Gupta G., Garcia A. E., Guo Q., Lu M., Kallenbach N. R. Structure of a parallel-stranded tetramer of the Oxytricha telomeric DNA sequence dT4G4. Biochemistry. 1993 Jul 20;32(28):7098–7103. doi: 10.1021/bi00079a005. [DOI] [PubMed] [Google Scholar]
- Guschlbauer W., Chantot J. F., Thiele D. Four-stranded nucleic acid structures 25 years later: from guanosine gels to telomer DNA. J Biomol Struct Dyn. 1990 Dec;8(3):491–511. doi: 10.1080/07391102.1990.10507825. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack M. C., Dobrinski B., Lurz R., Docherty K., Kilpatrick M. W. The human insulin gene linked polymorphic region exhibits an altered DNA structure. Nucleic Acids Res. 1992 Jan 25;20(2):231–236. doi: 10.1093/nar/20.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin C. C., Corregan M., Brown B. A., 2nd, Frederick L. N. Cytosine-cytosine+ base pairing stabilizes DNA quadruplexes and cytosine methylation greatly enhances the effect. Biochemistry. 1993 Jun 8;32(22):5870–5880. doi: 10.1021/bi00073a021. [DOI] [PubMed] [Google Scholar]
- Hardin C. C., Watson T., Corregan M., Bailey C. Cation-dependent transition between the quadruplex and Watson-Crick hairpin forms of d(CGCG3GCG). Biochemistry. 1992 Jan 28;31(3):833–841. doi: 10.1021/bi00118a028. [DOI] [PubMed] [Google Scholar]
- Henderson E., Hardin C. C., Walk S. K., Tinoco I., Jr, Blackburn E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987 Dec 24;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Huizenga D. E., Szostak J. W. A DNA aptamer that binds adenosine and ATP. Biochemistry. 1995 Jan 17;34(2):656–665. doi: 10.1021/bi00002a033. [DOI] [PubMed] [Google Scholar]
- Kang C., Zhang X., Ratliff R., Moyzis R., Rich A. Crystal structure of four-stranded Oxytricha telomeric DNA. Nature. 1992 Mar 12;356(6365):126–131. doi: 10.1038/356126a0. [DOI] [PubMed] [Google Scholar]
- Kettani A., Kumar R. A., Patel D. J. Solution structure of a DNA quadruplex containing the fragile X syndrome triplet repeat. J Mol Biol. 1995 Dec 8;254(4):638–656. doi: 10.1006/jmbi.1995.0644. [DOI] [PubMed] [Google Scholar]
- Kypr J., Chládková J., Arnold L., Sági J., Szemzö A., Vorlícková M. The unusual X-form DNA in oligodeoxynucleotides: dependence of stability on the base sequence and length. J Biomol Struct Dyn. 1996 Jun;13(6):999–1006. doi: 10.1080/07391102.1996.10508914. [DOI] [PubMed] [Google Scholar]
- Laughlan G., Murchie A. I., Norman D. G., Moore M. H., Moody P. C., Lilley D. M., Luisi B. The high-resolution crystal structure of a parallel-stranded guanine tetraplex. Science. 1994 Jul 22;265(5171):520–524. doi: 10.1126/science.8036494. [DOI] [PubMed] [Google Scholar]
- Lu M., Guo Q., Kallenbach N. R. Thermodynamics of G-tetraplex formation by telomeric DNAs. Biochemistry. 1993 Jan 19;32(2):598–601. doi: 10.1021/bi00053a027. [DOI] [PubMed] [Google Scholar]
- MacLeod M. C., Johnston D. A., LaBate M., White R. A. The probability of occurrence of oligomer motifs in the human genome and genomic microheterogeneity. J Theor Biol. 1996 Aug 21;181(4):311–318. doi: 10.1006/jtbi.1996.0133. [DOI] [PubMed] [Google Scholar]
- Macaya R. F., Schultze P., Smith F. W., Roe J. A., Feigon J. Thrombin-binding DNA aptamer forms a unimolecular quadruplex structure in solution. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3745–3749. doi: 10.1073/pnas.90.8.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaya R. F., Waldron J. A., Beutel B. A., Gao H., Joesten M. E., Yang M., Patel R., Bertelsen A. H., Cook A. F. Structural and functional characterization of potent antithrombotic oligonucleotides possessing both quadruplex and duplex motifs. Biochemistry. 1995 Apr 4;34(13):4478–4492. doi: 10.1021/bi00013a041. [DOI] [PubMed] [Google Scholar]
- Marck C., Thiele D. Poly(dG).poly(dC) at neutral and alkaline pH: the formation of triple stranded poly(dG).poly(dG).poly(dC). Nucleic Acids Res. 1978 Mar;5(3):1017–1028. doi: 10.1093/nar/5.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh T. C., Henderson E. G-wires: self-assembly of a telomeric oligonucleotide, d(GGGGTTGGGG), into large superstructures. Biochemistry. 1994 Sep 6;33(35):10718–10724. doi: 10.1021/bi00201a020. [DOI] [PubMed] [Google Scholar]
- Marsh T. C., Vesenka J., Henderson E. A new DNA nanostructure, the G-wire, imaged by scanning probe microscopy. Nucleic Acids Res. 1995 Feb 25;23(4):696–700. doi: 10.1093/nar/23.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie A. I., Lilley D. M. Retinoblastoma susceptibility genes contain 5' sequences with a high propensity to form guanine-tetrad structures. Nucleic Acids Res. 1992 Jan 11;20(1):49–53. doi: 10.1093/nar/20.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickol J. M., Felsenfeld G. DNA conformation at the 5' end of the chicken adult beta-globin gene. Cell. 1983 Dec;35(2 Pt 1):467–477. doi: 10.1016/0092-8674(83)90180-0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K., Padmanabhan K. P., Ferrara J. D., Sadler J. E., Tulinsky A. The structure of alpha-thrombin inhibited by a 15-mer single-stranded DNA aptamer. J Biol Chem. 1993 Aug 25;268(24):17651–17654. doi: 10.2210/pdb1hut/pdb. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988 Jul 28;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Novel DNA superstructures formed by telomere-like oligomers. Biochemistry. 1992 Jan 14;31(1):65–70. doi: 10.1021/bi00116a011. [DOI] [PubMed] [Google Scholar]
- Smith F. W., Feigon J. Quadruplex structure of Oxytricha telomeric DNA oligonucleotides. Nature. 1992 Mar 12;356(6365):164–168. doi: 10.1038/356164a0. [DOI] [PubMed] [Google Scholar]
- Sundquist W. I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989 Dec 14;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Traub P., Mothes E., Shoeman R. L., Schröder R., Scherbarth A. Binding of nucleic acids to intermediate filaments of the vimentin type and their effects on filament formation and stability. J Biomol Struct Dyn. 1992 Dec;10(3):505–531. doi: 10.1080/07391102.1992.10508665. [DOI] [PubMed] [Google Scholar]
- Usdin K., Woodford K. J. CGG repeats associated with DNA instability and chromosome fragility form structures that block DNA synthesis in vitro. Nucleic Acids Res. 1995 Oct 25;23(20):4202–4209. doi: 10.1093/nar/23.20.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venczel E. A., Sen D. Synapsable DNA. J Mol Biol. 1996 Mar 29;257(2):219–224. doi: 10.1006/jmbi.1996.0157. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Subirana J. A., Chládková J., Tejralová I., Huynh-Dinh T., Arnold L., Kypr J. Comparison of the solution and crystal conformations of (G + C)-rich fragments of DNA. Biophys J. 1996 Sep;71(3):1530–1538. doi: 10.1016/S0006-3495(96)79355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Y., Krawczyk S. H., Bischofberger N., Swaminathan S., Bolton P. H. The tertiary structure of a DNA aptamer which binds to and inhibits thrombin determines activity. Biochemistry. 1993 Oct 26;32(42):11285–11292. doi: 10.1021/bi00093a004. [DOI] [PubMed] [Google Scholar]
- Wang Y., Patel D. J. Solution structure of a parallel-stranded G-quadruplex DNA. J Mol Biol. 1993 Dec 20;234(4):1171–1183. doi: 10.1006/jmbi.1993.1668. [DOI] [PubMed] [Google Scholar]
- Wang Z., Lin X. H., Qiu Q. Q., Deuel T. F. Modulation of transcription of the platelet-derived growth factor A-chain gene by a promoter region sensitive to S1 nuclease. J Biol Chem. 1992 Aug 25;267(24):17022–17031. [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Wolk S., Thurmes W. N., Ross W. S., Hardin C. C., Tinoco I., Jr Conformational analysis of d(C3G3), a B-family duplex in solution. Biochemistry. 1989 Mar 21;28(6):2452–2459. doi: 10.1021/bi00432a016. [DOI] [PubMed] [Google Scholar]
- Woodford K. J., Howell R. M., Usdin K. A novel K(+)-dependent DNA synthesis arrest site in a commonly occurring sequence motif in eukaryotes. J Biol Chem. 1994 Oct 28;269(43):27029–27035. [PubMed] [Google Scholar]
- Wyatt J. R., Vickers T. A., Roberson J. L., Buckheit R. W., Jr, Klimkait T., DeBaets E., Davis P. W., Rayner B., Imbach J. L., Ecker D. J. Combinatorially selected guanosine-quartet structure is a potent inhibitor of human immunodeficiency virus envelope-mediated cell fusion. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1356–1360. doi: 10.1073/pnas.91.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]


