Abstract
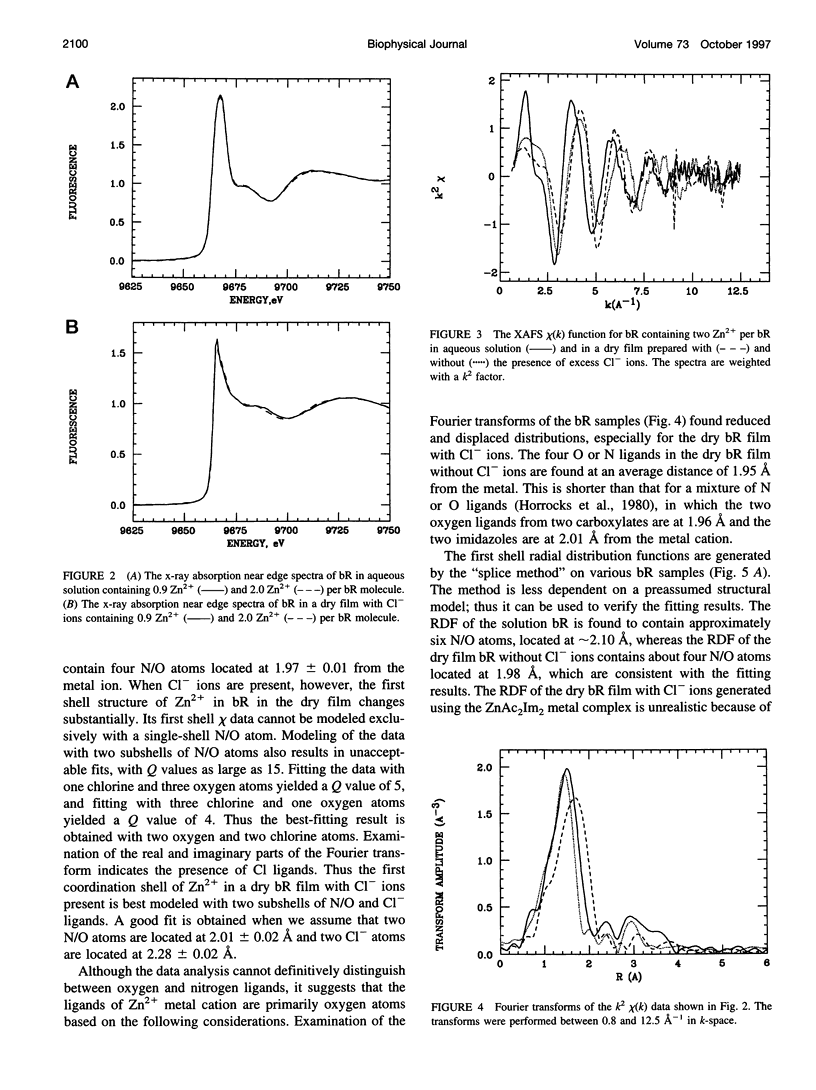
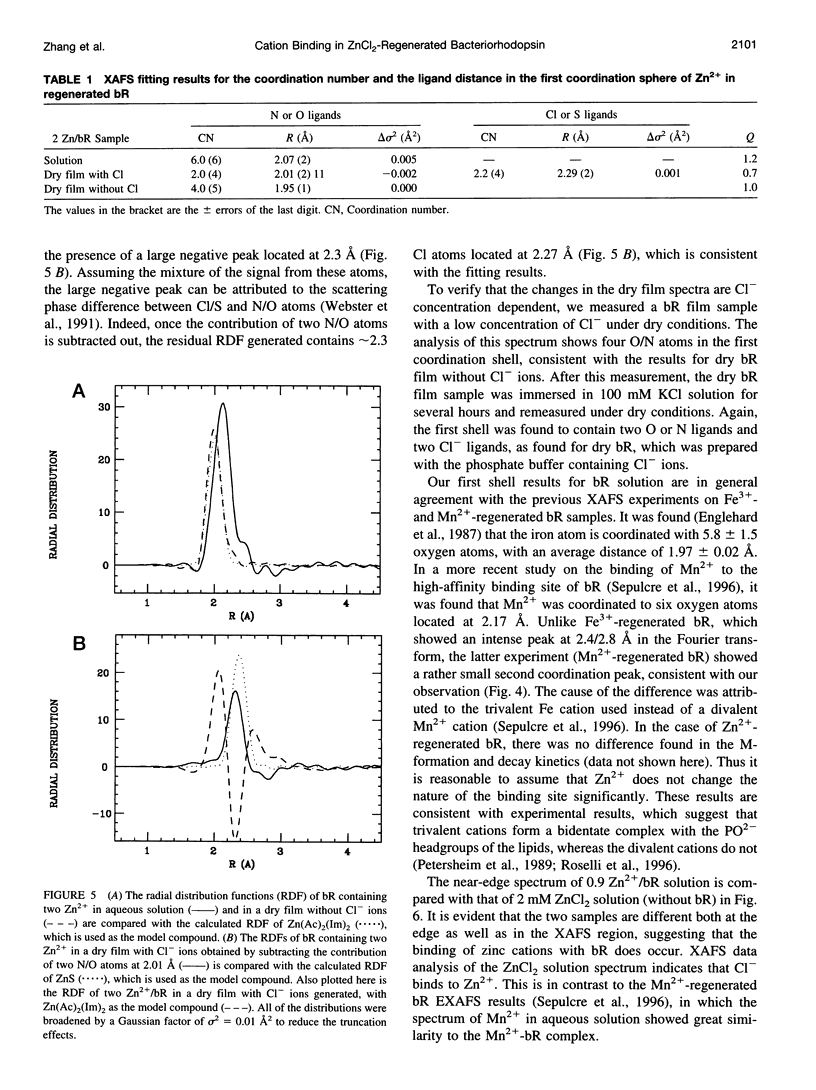
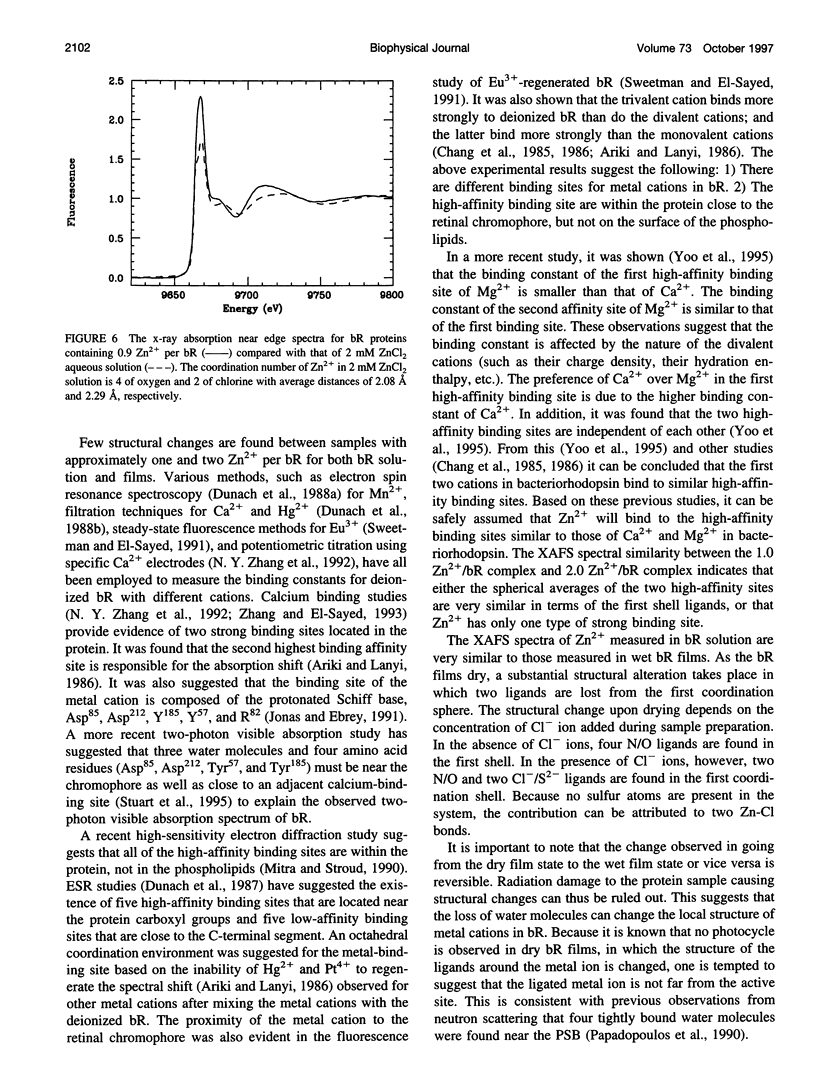
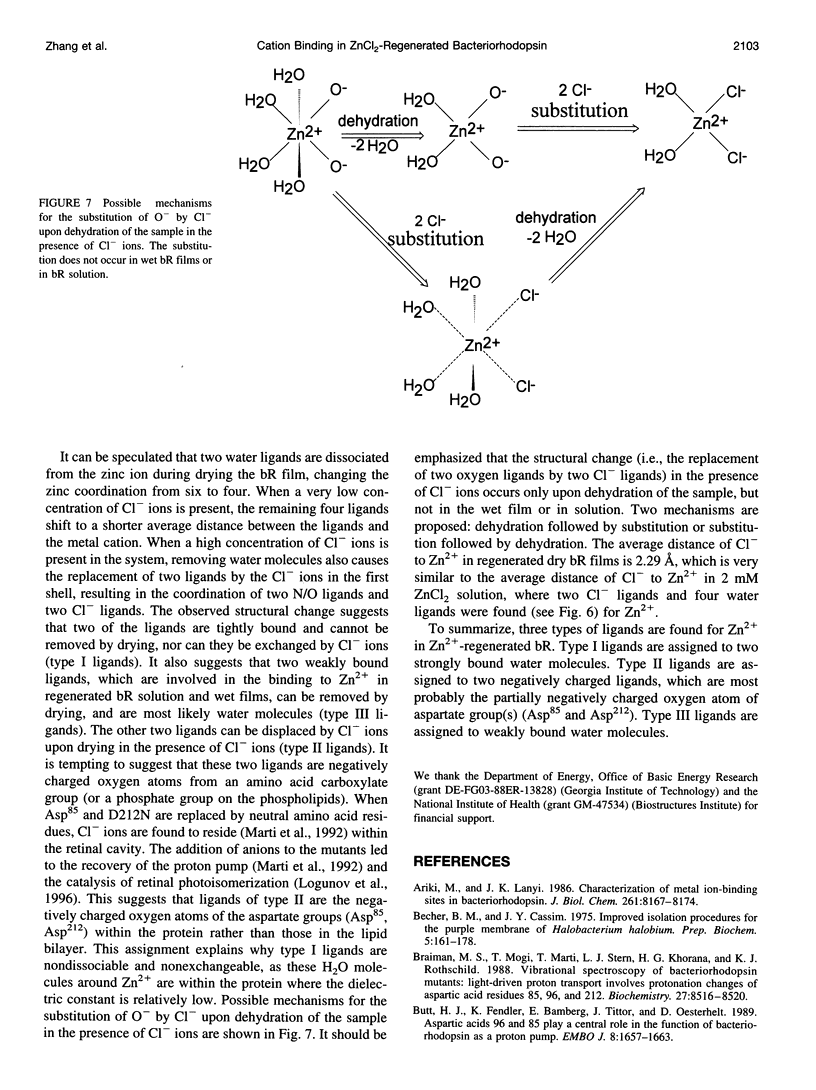
The binding of Zn2+ in Zn2+-regenerated bacteriorhodopsin (bR) was studied under various conditions by x-ray absorption fine structures (XAFS). The 0.9:1 and 2:1 Zn2+:bR samples gave similar XAFS spectra, suggesting that Zn2+ might have only one strong binding site in bR. It was found that in aqueous bR solution, Zn2+ has an average of six oxygen or nitrogen ligands. Upon drying, two ligands are lost, suggesting the existence of two weakly bound water ligands near the cation-binding site in bacteriorhodopsin. When excess Cl- ions were present before drying in the Zn2+-regenerated bR samples, it was found that two of the ligands were replaced by Cl- ions in the dried film, whereas two remain unchanged. The above observations suggest that Zn2+ has three types of ligands in regenerated bR (referred to as types I, II, and III). Type I ligands are strongly bound. These ligands cannot be removed by drying or by exchanging with Cl- ions. Type II ligands cannot be removed by drying, but can be replaced by Cl- ligands. Type III ligands are weakly bound to the metal cation and are most likely water molecules that can be removed by evaporation under vacuum or by drying with anhydrous CaSO4. The results are discussed in terms of the possible structure of the strongly binding site of Zn2+ in bR.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariki M., Lanyi J. K. Characterization of metal ion-binding sites in bacteriorhodopsin. J Biol Chem. 1986 Jun 25;261(18):8167–8174. [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Jonas R., Melchiore S., Govindjee R., Ebrey T. G. Mechanism and role of divalent cation binding of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):731–739. doi: 10.1016/S0006-3495(86)83699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronister E. L., Corcoran T. C., Song L., El-Sayed M. A. On the molecular mechanisms of the Schiff base deprotonation during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8580–8584. doi: 10.1073/pnas.83.22.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran T. C., Ismail K. Z., El-Sayed M. A. Evidence for the involvement of more than one metal cation in the Schiff base deprotonation process during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4094–4098. doi: 10.1073/pnas.84.12.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Ottolenghi M., Pande A., Pande J., Callender R. H. Acid-base equilibrium of the Schiff base in bacteriorhodopsin. Biochemistry. 1982 Sep 28;21(20):4953–4959. doi: 10.1021/bi00263a019. [DOI] [PubMed] [Google Scholar]
- Duñach M., Padrós E., Seigneuret M., Rigaud J. L. On the molecular mechanism of the blue to purple transition of bacteriorhodopsin. UV-difference spectroscopy and electron spin resonance studies. J Biol Chem. 1988 Jun 5;263(16):7555–7559. [PubMed] [Google Scholar]
- Duñach M., Seigneuret M., Rigaud J. L., Padrós E. Influence of cations on the blue to purple transition of bacteriorhodopsin. Comparison of Ca2+ and Hg2+ binding and their effect on the surface potential. J Biol Chem. 1988 Nov 25;263(33):17378–17384. [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Surface-bound optical probes monitor protein translocation and surface potential changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5996–6000. doi: 10.1073/pnas.89.13.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks W. D., Jr, Ishley J. N., Holmquist B., Thompson J. S. Structural and electronic mimics of the active site of cobalt(II)-substituted zinc metalloenzymes. J Inorg Biochem. 1980 Apr;12(2):131–141. doi: 10.1016/s0162-0134(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Jonas R., Ebrey T. G. Binding of a single divalent cation directly correlates with the blue-to-purple transition in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):149–153. doi: 10.1073/pnas.88.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katre N. V., Kimura Y., Stroud R. M. Cation binding sites on the projected structure of bacteriorhodopsin. Biophys J. 1986 Aug;50(2):277–284. doi: 10.1016/S0006-3495(86)83461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov S. L., el-Sayed M. A., Lanyi J. K. Catalysis of the retinal subpicosecond photoisomerization process in acid purple bacteriorhodopsin and some bacteriorhodopsin mutants by chloride ions. Biophys J. 1996 Sep;71(3):1545–1553. doi: 10.1016/S0006-3495(96)79357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Marti T., Otto H., Rösselet S. J., Heyn M. P., Khorana H. G. Anion binding to the Schiff base of the bacteriorhodopsin mutants Asp-85----Asn/Asp-212----Asn and Arg-82----Gln/Asp-85----Asn/Asp-212----Asn. J Biol Chem. 1992 Aug 25;267(24):16922–16927. [PubMed] [Google Scholar]
- Mitra A. K., Stroud R. M. High sensitivity electron diffraction analysis. A study of divalent cation binding to purple membrane. Biophys J. 1990 Feb;57(2):301–311. doi: 10.1016/S0006-3495(90)82532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustre de Leon J, Rehr JJ, Zabinsky SI, Albers RC. Ab initio curved-wave x-ray-absorption fine structure. Phys Rev B Condens Matter. 1991 Sep 1;44(9):4146–4156. doi: 10.1103/physrevb.44.4146. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. Flash-induced volume changes of bacteriorhodopsin-containing membrane fragments and their relationship to proton movements and absorbance transients. J Biol Chem. 1978 Sep 10;253(17):6158–6164. [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos G., Dencher N. A., Zaccai G., Büldt G. Water molecules and exchangeable hydrogen ions at the active centre of bacteriorhodopsin localized by neutron diffraction. Elements of the proton pathway? J Mol Biol. 1990 Jul 5;214(1):15–19. doi: 10.1016/0022-2836(90)90140-h. [DOI] [PubMed] [Google Scholar]
- Petersheim M., Halladay H. N., Blodnieks J. Tb3+ and Ca2+ binding to phosphatidylcholine. A study comparing data from optical, NMR, and infrared spectroscopies. Biophys J. 1989 Sep;56(3):551–557. doi: 10.1016/S0006-3495(89)82702-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli C., Boussac A., Mattioli T. A., Griffiths J. A., el-Sayed M. A. Detection of a Yb3+ binding site in regenerated bacteriorhodopsin that is coordinated with the protein and phospholipid head groups. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14333–14337. doi: 10.1073/pnas.93.25.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre F., Cladera J., García J., Proietti M. G., Torres J., Padrós E. An extended x-ray absorption fine structure study of the high-affinity cation-binding site in the purple membrane. Biophys J. 1996 Feb;70(2):852–856. doi: 10.1016/S0006-3495(96)79627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheves M., Albeck A., Friedman N., Ottolenghi M. Controlling the pKa of the bacteriorhodopsin Schiff base by use of artificial retinal analogues. Proc Natl Acad Sci U S A. 1986 May;83(10):3262–3266. doi: 10.1073/pnas.83.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Ma Y, Hanske-Petitpierre O, Bouldin CE. Radial distribution function in x-ray-absorption fine structure. Phys Rev B Condens Matter. 1992 Jul 1;46(2):687–694. doi: 10.1103/physrevb.46.687. [DOI] [PubMed] [Google Scholar]
- Sweetman L. L., el-Sayed M. A. The binding site of the strongly bound Eu3+ in Eu(3+)-regenerated bacteriorhodopsin. FEBS Lett. 1991 May 6;282(2):436–440. doi: 10.1016/0014-5793(91)80531-7. [DOI] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Effect of lipid surface charges on the purple-to-blue transition of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3681–3684. doi: 10.1073/pnas.84.11.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Purple-to-blue transition of bacteriorhodopsin in a neutral lipid environment. Biophys J. 1988 Aug;54(2):227–232. doi: 10.1016/S0006-3495(88)82951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Surface pH controls purple-to-blue transition of bacteriorhodopsin. A theoretical model of purple membrane surface. Biophys J. 1989 Aug;56(2):369–383. doi: 10.1016/S0006-3495(89)82683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster L. C., Zhang K., Chance B., Ayene I., Culp J. S., Huang W. J., Wu F. Y., Ricciardi R. P. Conversion of the E1A Cys4 zinc finger to a nonfunctional His2,Cys2 zinc finger by a single point mutation. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9989–9993. doi: 10.1073/pnas.88.22.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Chance B., Auld D. S., Larsen K. S., Vallee B. L. X-ray absorption fine structure study of the active site of zinc and cobalt carboxypeptidase A in their solution and crystalline forms. Biochemistry. 1992 Feb 4;31(4):1159–1168. doi: 10.1021/bi00119a027. [DOI] [PubMed] [Google Scholar]
- Zhang K., Stern E. A., Ellis F., Sanders-Loehr J., Shiemke A. K. The active site of hemerythrin as determined by X-ray absorption fine structure. Biochemistry. 1988 Sep 20;27(19):7470–7479. doi: 10.1021/bi00419a045. [DOI] [PubMed] [Google Scholar]
- Zhang N. Y., el-Sayed M. A. The C-terminus and the Ca2+ low-affinity binding sites in bacteriorhodopsin. Biochemistry. 1993 Dec 28;32(51):14173–14175. doi: 10.1021/bi00214a015. [DOI] [PubMed] [Google Scholar]
- Zhang Y. N., Sweetman L. L., Awad E. S., El-Sayed M. A. Nature of the individual Ca binding sites in Ca-regenerated bacteriorhodopsin. Biophys J. 1992 May;61(5):1201–1206. doi: 10.1016/S0006-3495(92)81929-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. N., el-Sayed M. A., Bonet M. L., Lanyi J. K., Chang M., Ni B., Needleman R. Effects of genetic replacements of charged and H-bonding residues in the retinal pocket on Ca2+ binding to deionized bacteriorhodopsin. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1445–1449. doi: 10.1073/pnas.90.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]


