Abstract
Heterozygous β-globin gene knockout thalassemia (BKO) mice derived from C57BL/6 wild-type (WT) mice have phenotypic of β-thalassemia (BT) and have been widely used for studying this disease except reproductive disorders. The present study determined whether male BKO mice recapitulate reproductive problems as BT men. Mice were randomly assigned into groups depending on the genotype (WT vs. BKO) and intervention (control vs. iron-loaded). Euthanized mice were collected blood, testes, epididymides, hypothalamus, and anterior pituitary for assessing hematological parameters, plasma iron and testosterone levels, testis iron levels, sperm characteristics, and histological alterations. Iron administration caused significant increases of plasma and testis iron levels (p < 0.001) but had no significant influence on the hematological profile of BKO mice, which indeed had fewer erythrocyte, hemoglobin, and hematocrit but had greater reticulocyte than WT (p < 0.001 to p = 0.017). Furthermore, irrespective of the genotype, iron administration decreased plasma testosterone levels (p = 0.03 to p > 0.05), total sperm count (p < 0.001), and percent normal sperm morphology (p ≤ 0.01). Based on Perls’ Prussian blue staining, excess iron was ubiquitously present in the anterior pituitary and testicular interstitium of iron-loaded mice. This mineral, however, caused no significant changes in reproductive organs microstructure as visualized by hematoxylin and eosin staining. In conclusion, besides physiological dysfunction of many organ systems, iron-loaded male BKO mice exhibit reproductive problems and abnormal sperm characteristics similar to BT men. Therefore, this animal model seems invaluable for future biomedical research involved in various aspects of BT-related male reproductive disorders.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87619-y.
Keywords: β-thalassemia, Hypogonadism, Iron overload, Mouse, Testicular histology, Sperm characteristics
Subject terms: Systems biology, Zoology, Diseases
Introduction
β-thalassemia (BT) is an inherited blood disorder caused by changes in the β-globin gene. This disease causes the body to make fewer, abnormal hemoglobin and therefore results in anemia. BT comprises three main types depending on the number of β-globin genes affected and the severity of anemia: (I) BT major, which is the most severe form of this disorder that demonstrates severe anemia, slowed growth, yellowish skin, enlarged spleen, liver, and heart, as well as brittle bones1; people with BT major require regular blood transfusions and iron-lowering medications to sustain their life; (II) BT minor, which causes asymptomatic to mild symptoms of anemia; and (III) BT intermedia, which has a diverse clinical spectrum (a clinical polymorphism) spanning from mild to moderate anemia, and individuals with this condition may require occasional transfusions2. Globally, BT major constitutes about 1.5% of the total population with the majority living in developing countries3. BT minor accounts for approximately 3% of the global population4; the Mediterranean, Middle and Far East, Africa, Central and Southeast Asia, and the Indian subcontinent are among the most affected areas5. Up to one-fourth of BT patients throughout the world are represented by BT intermedia6.
Iron built up in the BT patient organs from blood transfusions and increased gastrointestinal absorption can interfere with a normal function of such organs including those in the reproductive system. Several studies have reported that as many as 80% of male BT major patients have delayed puberty, lack of pubertal development, infertility, and sexual dysfunction7,8. On the contrary, almost 70% of BT intermedia males have normal seminal parameters and only 30% encounter low sperm count and/or motility9. The reproductive problems observed in BT men, regardless of the type, are associated with the hypothalamic-pituitary-gonadal (HPG) axis disorders and are largely attributed to the damage caused by chronic anemia and the deposition of iron in the brain and testes. Excess iron deposition in the hypothalamus and/or pituitary leads to gonadotropin secretory dysfunction and hence a lack of sex hormones. At the male gonads, iron deposition renders histomorphological alterations10,11 and contributes to the poor semen quality12,13.
House mice (Mus musculus) have long been used as model organisms for studying human biology and disease by reasons of, for instance, the genetic homologies and physiological similarities between the two species and the ease of breeding and maintaining them in the animal facility14–16. The development of methods for the generation of transgenic, knockout, and knockin mice has provided powerful tools for mouse research and has led to a substantial increase in the use of mice as models for human. Heterozygous β-globin gene knockout thalassemia (BKO) mice derived from inbred C57BL/6 mice were generated by heterozygous deletion of both murine β-globin genes (Hbb-b1 and Hbb-b2)17,18. Phenotypic characterization of this mouse model resembles clinical features of BT intermedia patients, namely mild to moderate anemia, growth retardation, ineffective erythropoiesis, and iron accumulation in several tissues such as heart, spleen, and liver18. Thus, these mice have been pervasively used as models for studying various aspects of this disease including red blood cell pathology19, iron-overload cardiomyopathy20, osteoporosis21, infection22, abnormal glucose metabolism21, and the novel therapeutic approaches23. However, it is worth noting that BKO mice have never been used to study reproductive disorders caused by the disease, although reproductive health impairment is also a complication that can occur in BT individuals8,9,12. Therefore, understanding sperm characteristics in BKO mice may be useful for further studies on semen problems in male patients. The present study aimed to determine whether the BKO mice recapitulate reproductive problems as BT men. Sperm characteristics of C57BL/6 wild-type (WT) and BKO mice with or without iron loading were examined. Hematological and pathological alterations and iron accumulation in the mice blood, brain, and testes were also studied.
Results
Iron administration increased plasma iron levels but did not alter hematological profiles of mice
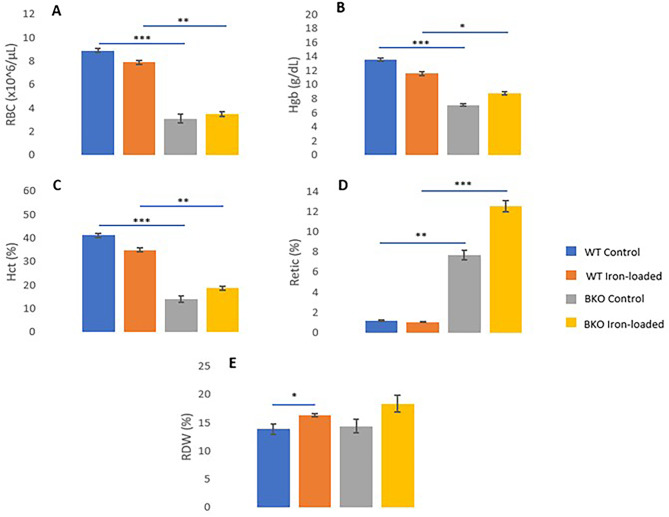
As the levels of iron in plasma were comparable between WT control and BKO control mice, halves of the mice in the present study were induced hemochromatosis to simulate BT patients’ conditions. After four weeks of iron administration, the levels of iron in plasma increased significantly (i.e., 15- to 16-fold) in both WT and BKO mice compared to those of genotype-matched controls (p < 0.001) (Fig. 1A and Supplementary Table 1). The average plasma iron levels in WT iron-loaded mice did not differ from the values in BKO iron-loaded animals (p > 0.05). However, it was very interesting that iron administration had no significant influence on hematological parameters except for red cell distribution width (RDW) in WT mice that exogenous iron resulted in a significant increase of the value (RDW of 16.36 ± 0.22% in iron-loaded mice vs. 13.89 ± 0.93% in control mice, p = 0.001). Comparing the two genotypes, drastic decreases of red blood cell count (RBC), total hemoglobin (Hgb), and of hematocrit (Hct) (p < 0.001 to p = 0.017) and a substantial increase of Reticulocyte count (Retic) (p < 0.001 to p = 0.001) were observed in BKO mice whether or not iron was administered (Fig. 2).
Fig. 1.
Plasma levels of iron (A) and testosterone (B) in male WT and BKO mice with and without iron loading. × represents mean values. The statistical analysis was conducted using (A) the Kruskal–Wallis test and (B) one-way ANOVA, and significant differences were found at p < 0.001 (***) and p < 0.05 (*).
Fig. 2.
Comparisons of hematological parameters between WT and BKO mice with and without iron administration. A Red blood cell count, B total hemoglobin, C hematocrit, D reticulocyte count, and E red cell distribution width. Values are represented as mean ± SEM. Significant differences were found at p < 0.001 (***), p < 0.01 (**), and p < 0.05 (*).
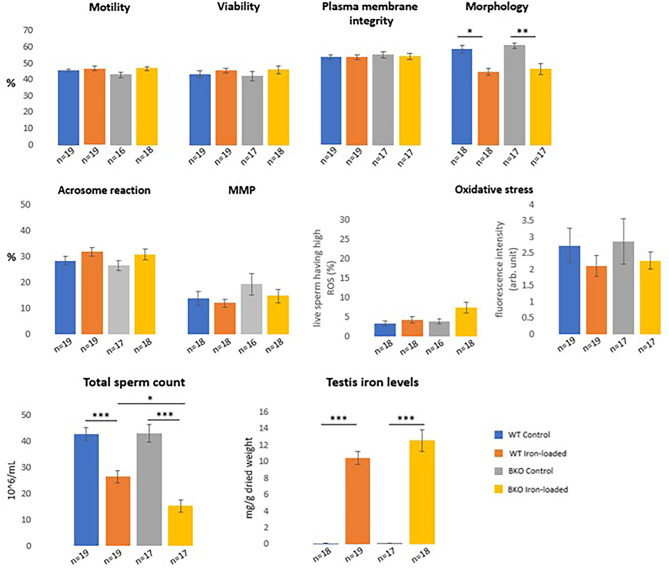
Iron administration increased testis iron levels but decreased in vitro sperm quality
Similar to plasma, iron administration resulted in a dramatic increase in the levels of iron in the mice testis determined by a colorimetric method (i.e., 80- to 90-fold increases for WT and BKO mice, p < 0.001) (Fig. 3 and Supplementary Table 1). The average iron levels in testis of BKO mice were not significantly different from the values of WT mice both before and after iron injection (p > 0.05).
Fig. 3.
Sperm characteristics and testis iron levels in WT and BKO mice with and without iron administration (means ± SEM). The statistical analysis was conducted using one-way ANOVA (total sperm count, viability, plasma membrane integrity, acrosome reaction, and percentage of viable sperm with high ROS) and Kruskal–Wallis test (motility, normal sperm morphology, mitochondrial membrane potential (MMP), fluorescence intensity, and testis iron levels). Significant differences were found at p < 0.001 (***), p < 0.01 (**), and p < 0.05 (*).
Epididymal sperm characteristics of iron-loaded mice, regardless of the genotype, were inferior to those of the controls in the aspect of sperm numbers (p < 0.001) and proportion of morphological normal spermatozoa (p < 0.01 to p = 0.01); and among iron-loaded animals, a decrease in sperm numbers was more noticeable and severe in BKO mice (15.24 ± 2.39 × 106/mL) than WT mice (26.42 ± 2.44 × 106/mL, p = 0.02). Intriguingly, the motility, viability, membrane integrity, acrosomal status, mitochondrial membrane potential, and oxidative stress of spermatozoa were not affected by the mouse genotype and exogenous iron treatment (all were p > 0.05) (Fig. 3 and Supplementary Table 2).
Altered sperm characteristics were related to the levels of iron and testosterone
To determine whether decreased sperm count and increased abnormal sperm morphology in iron-loaded mice were associated with elevated iron levels, we used a Spearman’s rank correlation and found the inverse relationship between iron levels and total sperm count, with Spearman’s rank correlation coefficient (rs) of − 0.68 and − 0.71 for plasma iron and testis iron, respectively (p < 0.001) (Fig. 4A, B). A significant inverse correlation was also demonstrated in percent normal sperm morphology with rs = − 0.63 for plasma iron and rs = − 0.67 for testis iron (both were p < 0.001) (Fig. 4C, D). Furthermore, testis iron levels were found to be directly correlated to the percentage of viable spermatozoa having high oxidative stress (rs = 0.37, p = 0.01) (Fig. 4E). These confirmed that iron accumulation adversely affected sperm characteristics of male mice. In theory, oxidative stress is detrimental to sperm plasma and acrosomal membranes due to the high content of polyunsaturated fatty acids (PUFAs) in the membranes, resulting in disruption of membrane permeability, diminished acrosomal function, impaired sperm motility, and probably infertility24–26. Also, oxidative stress can cause sperm DNA damage, resulting in the passage of defective paternal DNA on to the conceptus27.
Fig. 4.
Spearman’s rank correlation coefficient (rs) analysis between the levels of iron and testosterone and sperm parameters in male mice. Significant relationships between (A) plasma iron levels and total sperm count (n = 69), B testis iron levels and total sperm count (n = 72), C plasma iron levels and percent normal sperm morphology (n = 68), D testis iron levels and percent normal sperm morphology (n = 70), E testis iron levels and percent viable sperm having high ROS (n = 70), and F plasma testosterone levels and total sperm count (n = 49).
In the present study, total sperm count was the sole sperm parameter significantly associated with plasma levels of testosterone (rs = 0.32, p = 0.03) (Fig. 4F), which plasma testosterone values were lower in male mice injected by iron dextran compared with their counterparts received no injection (p > 0.05 for WT and p = 0.03 for BKO mice) (Fig. 1B and Supplementary Table 2).
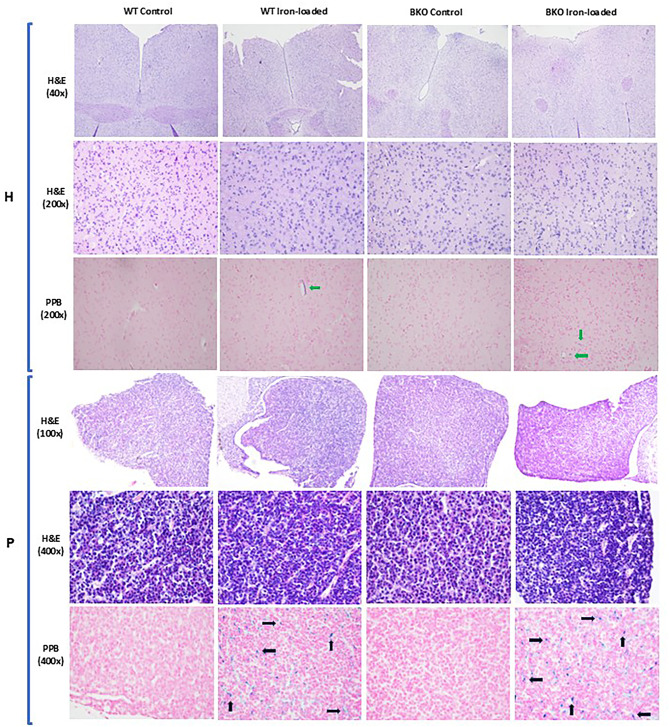
Excessive iron deposited in but did not change microstructures of the HPG axis
In animals, the HPG axis plays a critical part in the development and regulation of several body’s systems including the reproductive system. The perturbation of this axis can lead to changes in its structure and/or function and has various local and systemic effects on the body. In the present study, histological morphology of the hypothalamus, anterior pituitary, and testis of WT and BKO mice with and without iron loading was observed in the hematoxylin and eosin (H&E)-stained slides, and no significant changes were found in iron-loaded mice organs compared to genotype-matched control groups. In all four groups, the large neurons located in the hypothalamus were interspersed with numerous astrocytes; the parenchyma of the anterior pituitary was observed with clusters of neuronal cells mingled with connective tissue and sinusoidal capillaries (Fig. 5). Microstructure of seminiferous tubules in the testis of WT control and BKO control mice was not significantly different, i.e., mean Johnsen scores of 3.24 ± 0.19 and 3.49 ± 0.07, respectively (p > 0.05). Almost all seminiferous tubules observed comprised a number of germ cells lined from the basement membrane to the lumen with a relatively constant thickness. The long, thin heads of mature spermatozoa were also evident in many tubules especially the adluminal compartment indicating spermatogenesis successfully occurred in these males (Fig. 6). Such promising pictures could also be seen in the seminiferous tubules of WT and BKO mice treated with 200 mg iron dextran, with mean Johnsen scores of 3.19 ± 0.18 for WT iron-loaded and 3.28 ± 0.14 for BKO iron-loaded mice (p > 0.05).
Fig. 5.
Histological structure of the hypothalamus (H, 40× and 200× magnifications) and anterior pituitary (P, 100× and 400× magnifications) of WT and BKO mice visualized by hematoxylin and eosin (H&E) and Perls’ Prussian blue (PPB) staining. The excessive accumulation of iron in the hypothalamus (green arrows) and the anterior pituitary (black arrows) was observed.
Fig. 6.
Histological structure of the seminiferous tubules in the testis (40× and 100× magnifications) of WT and BKO mice visualized by hematoxylin and eosin (H&E) and Perls’ Prussian blue (PPB) staining. The excessive accumulation of iron in the testis (red arrows) was observed. Inset is an example of the seminiferous tubule containing many mature spermatozoa.
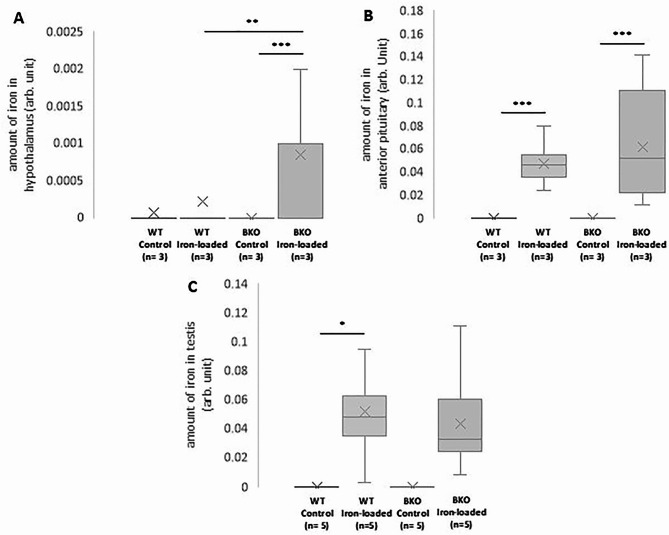
The deposition of iron in the hypothalamus, anterior pituitary, and testis was visualized by PPB staining. The tissues containing iron will theoretically exhibit a blue hue in the PPB-stained section. It was found that the hypothalamus, anterior pituitary, and testis of iron-loaded mice, but not control mice, both WT and BKO, were deposited by iron in varying extents as bluish pigments of iron were visible (Figs. 5 and 6). Using computerized image analysis, the average amount of iron was greater in the hypothalamus and anterior pituitary of BKO iron-loaded mice compared to that of WT-iron loaded animals with a significant difference only noted in the hypothalamus (p = 0.005) (Fig. 7A, B). However, using an identical method, amount of iron present in the testis did not differ significantly between both groups (Fig. 7C).
Fig. 7.
Box plots of the distributions of the amount of iron deposited in the hypothalamus (A), anterior pituitary (B), and the testis (C) of male WT and BKO mice measured in Perls’ Prussian blue stained sections by computerized image analysis. × represents mean values. Significant differences were found at p < 0.001 (***), p < 0.01 (**), and p < 0.05 (*).
Discussion
Sperm characteristics in BKO mice were studied to answer a research question “do BKO mice have male reproductive problems similar to those found in BT patients?”. It was revealed that in vitro sperm characteristics in BKO control mice including but not limited to sperm count, motility, viability, normal morphology, and acrosomal status were all acceptable and identical to those in age-matched WT mice. This observation tentatively suggests that young adult male BKO mice that do not receive exogenous iron have no observable problems with their sperm quality and therefore seem not suitable for studying male reproductive disorders in BT patients. Indistinguishable sperm characteristics between WT and BKO mice were supported, partially or totally, by the fact that WT and BKO mice have the same genetic background (i.e., C57BL/6); male animals with different genetics produce sperm cells with different quality28,29; and that β-globin gene deletion in C57BL/6 WT mice per se, a technique to generate BKO mice, does not disrupt gene(s) regulating sperm production18.
The excessive accumulation of iron in different organs is frequently reported as a major consequence of BT, both transfusion-dependent thalassemia and non-transfusion-dependent thalassemia, and results in reactive oxygen species (ROS) formation followed by multisystemic complications30. In the present study, a total of 200 mg iron dextran was administered intraperitoneally to a half of male BKO mice in order to induce iron overload and hence to increase the chance of finding cellular oxidative stress and male reproductive problems. It was found that both WT and BKO mice injected with iron dextran had much increased plasma and testis iron levels compared to WT and BKO mice without injection, suggesting that exogenous iron dispersed systemically and deposited in various organs at least in the testis – a male reproductive gland that produces spermatozoa – of male mice. Our results agree with previous findings demonstrating an increase in the serum and testis iron levels in rats with an acute iron overload31,32. Regarding sperm characteristics, total sperm count and the percentage of normal sperm morphology in iron-loaded WT and BKO mice were considerably lower than those in control mice, and proportion of viable spermatozoa with high oxidative stress levels in these mice tended to be higher than normal males. These findings are similar to earlier studies that reported most of male BT patients with chronic blood transfusion having abnormal sperm characteristics including low sperm concentration and low normal sperm morphology33,34. The sperm abnormalities were possibly associated with, in the level of the gonads, disrupted Leydig cell function because of massive iron and ROS accumulations and impaired testosterone production and secretion. Such assumption was corroborated by significant relationships between testis iron levels and some sperm parameters (i.e., sperm count, normal morphology, and living cells with high ROS) as well as by a positive correlation between plasma testosterone levels and total sperm count, found in the present study. Testosterone is a male sex steroid hormone produced mainly by Leydig cells of the testis after being stimulated by luteinizing hormone (LH), secreted from gonadotrophic cells in the anterior pituitary, and gonadotropin-releasing hormone (GnRH), released from the hypothalamus35. This androgenic steroid is indispensable for secondary sexual characteristics, sperm cell production and development, and ultimately male fertility36. It is interesting that based on PPB staining, iron accumulation was sparingly observed in the hypothalamus and was remarkably found in the anterior pituitary and the interstitial space of the testis of iron-loaded WT and BKO mice. Our PPB staining results agree with the study of Rossi et al.32 which exploited optical emission spectrometry and found detectable amount of accumulated iron in the hypothalamus of iron-loaded female rats in a dose dependent fashion and also with previous findings which indicated that hypogonadism in males with chronic iron overload is caused by iron accumulation in gonadotropic cells of the anterior pituitary gland leading to inadequate pituitary responsiveness to GnRH37–39 and in interstitial Leydig cells of the testis leading to inefficient production of testosterone40. In the testis, levels of transferrin – a protein involved in iron transport – and its receptor (TfR1) were highest in Leydig cells but were very low in Sertoli cells and developing germ cells41,42, and the capacity of iron transport from circulation to germ cells is limited by the blood-testis barrier43. These could be potential reasons for histological findings in PPB-stained testis sections, which iron granules were mainly present in the interstitial compartment of the iron-loaded mice testes.
In the present study, histological morphology of the hypothalamic, pituitary, and testicular tissue sections stained with H&E did not differ significantly among groups. Irrespectively of the groups, the hypothalamus and anterior pituitary still contain a large number of intact neuronal cells and glial cells, and almost all of the seminiferous tubules in the testes still include a lot of germ cells with different developmental stages from spermatogonia to fully developed spermatozoa, that indicate complete spermatogenesis. No clear alterations in the histomorphology of the brain and the testis of iron-loaded mice corresponded to results of sperm characteristics and PPB staining which the latter demonstrated presence or absence of excess iron in some particular areas of the HPG axis.
Red blood cell indices were a sole parameter that differed apparently between mice genotypes whether or not exogenous iron was given. Compared with WT, male BKO mice had substantially decreased RBC, Hgb, and Hct. Reticulocyte count and RDW of such mice, however, tended to be or were significantly higher than those of normal controls. Decreases in RBC, Hgb, and Hct as well as an increase in Retic were aligned with the theory, recapitulated the clinical manifestation of BT patients, and were associated with ineffective erythropoiesis, chronic anemia, and iron overload2. Hematological alterations found herein were also depicted in recent studies which used the same mouse colony for experiments44,45.
Based on the results of the present study, it could be concluded that, apart from the physiological dysfunction of many organ systems such as hematologic, cardiovascular, hepatic, and neurologic that has been well addressed, male BKO mice treated with exogenous iron exhibit reproductive problems and abnormal sperm characteristics such as hypogonadism, oligospermia, and teratospermia. Spectrum of the severity of multisystemic, including male reproductive, disorders in BKO-iron loaded mice mostly resembles what has been reported in BT intermedia more than BT major. Consequently, these genetically modified mice are deemed to be a precious tool for studying multiple facets of male reproductive characteristics and/or disorders in BT intermedia patients in future biomedical research which include, for example, in vitro and in vivo fertility of spermatozoa and advanced infertility treatments.
Materials and methods
Animals, iron loading, and specimen collection
All animal housing and handling, the experimental techniques, and reporting of animal research were undertaken in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0 and relevant regulations concerning the use and care of experimental animals. This study was approved by the Animal Care and Use Committee at Institute of Molecular Biosciences (COA.NO.IMB-ACUC 2021/014).
Two-month-old male mice weighing approximately 25 g housed in polystyrene cages and maintained in a Strict Hygienic Conventional system at a temperature of 22 ± 2 °C, a relative humidity of 55 ± 15%, and a 12:12-hour light-dark cycle were randomly assigned following blocked randomization procedures (computer-generated random numbers) into four groups depending on the genotype (WT vs. BKO) and intervention (control vs. iron-loaded). Mice in the control groups received no intervention. Mice in the iron-loaded groups were injected intraperitoneally with iron dextran (10 mg/mouse) 5 days (Mon-Fri) per week for 4 weeks to induce increased absorption- and/or transfusion-associated iron overload, as described by Oudit et al.46. The clinical condition of all animals especially iron-loaded mice was monitored daily by animal care technicians and/or the Attending Veterinarian throughout the experimental period. At four-month-old, each mouse was euthanized by inhalation of an overdose of isoflurane (Arrane, Anaquest, Madison, WI 53713, USA). The mouse thoracic cavity was abruptly exposed, and blood was collected via the right ventricle using a 26-gauge needle. Then, the male reproductive organs (the testes and epididymides) and brain (the hypothalamus and anterior pituitary gland) were retrieved from the animal. The collected samples were further processed to evaluate, for example, iron levels, sex hormone levels, histological change, and sperm characteristics. A summary of the study design is shown in Fig. 8.
Fig. 8.
Flowchart of the study design.
Measurement of hematological and biochemical parameters
After euthanasia, mice blood (approximately 0.7 mL/mouse) was collected by cardiac puncture. An aliquot (50 µL) of freshly drawn heparin-treated blood was used for complete blood count. Hematological results were obtained with an automated hematological analyzer using the optical counting method (Mindray, Shenzhen, China).
For biochemical analyses, the rest of heparinized blood was centrifuged (1,500 × g, 10 min); the clear supernatant was gently aspirated, placed in microtubes, and stored deep-frozen (-80 °C) until processing. Plasma iron concentration, an indicator of iron deficiency or iron overload, was determined using an iron assay kit (BioAssay Systems, Hayward, CA, USA) according to the manufacturer’s protocol, which recommends not to include iron chelators such as ethylenediaminetetraacetic acid (EDTA) in sample preparation. Owing to very low plasma levels of testosterone in iron-loaded mice, the quantitative measurement of plasma testosterone was carried out by a competitive immunoenzymatic assay (ELISA) using the testosterone high sensitivity ELISA kit (ADI-901-176; Enzo Life Sciences Inc., Farmingdale, NY), which involves sample extraction before assaying to concentrate testosterone in the samples.
Measurement of sperm parameters
Spermatozoa collected from the epididymides of each mouse by cutting technique were released into 400 µL of sperm-TALP; the sperm sample placed in a microtube was always maintained at 37 °C in a water bath during sperm analysis. The sperm-TALP preparation and epididymal sperm collection method was described thoroughly elsewhere47.
Total sperm count
Motile spermatozoa were immobilized by diluting an aliquot of sperm suspension in formal saline [1:40 (v/v), respectively]. The total number of spermatozoa was subsequently determined using a hemocytometer counting chamber48 and calculated by the formula: total sperm count (× 106 cells/mL) = dilution factor × No. of sperm counted in 5 squares × 0.05 × 106.
Sperm motility
A drop of sperm suspension placed on a warm glass slide and covered with a cover slip was observed for total (progressive and non-progressive) sperm motility through a light microscope (× 400). The percentage of total motile spermatozoa was given from an average of ten fields of two slides.
Sperm viability
Spermatozoa were stained with a combination of eosin and nigrosin dyes49. Sperm viability examination was made in films smeared on a glass slide. Under high power (400× magnification) brightfield microscopy, a total of 200 spermatozoa per slide were counted, and the percentage of viable (unstained) cells was recorded.
Sperm plasma membrane integrity
An aliquot of sperm suspension was incubated, at 37 °C, with ten times the volume of the hypo-osmotic (75 mOsm/kg) solution50. After 20 min, spermatozoa with curled swollen tails that represent intact plasma membranes were observed under a light microscope (× 400). The percentage of membrane intact spermatozoa was calculated based on 200 total spermatozoa counted.
Sperm morphology
The quantitative evaluation of sperm morphology was achieved by Diff-Quik method51. After fixing, staining, and rinsing, the air-dried sperm smears were examined under the microscope (× 1,000). Two hundred spermatozoa were assessed for detailed abnormality pertaining to head, mid piece, and tail. Only the number of spermatozoa without abnormal morphology was noted in the results.
Acrosome reaction
The acrosomal status of mice spermatozoa was assessed using fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) ˗ a fluorescent dye that binds specifically to the outer acrosomal membrane and is used as a probe to visualize acrosomal integrity ˗ according to a method of Nagy et al.52 with some modifications. Briefly, a 120 µL of sperm suspension (2 × 106/mL) was incubated in the FITC-PNA solution [25.7 µM in phosphate-buffered saline (PBS)]. After 10 min, the membrane impermeant vital stain propidium iodide (PI; 74.8 µM) and 300 µL of PBS were additionally added to the mixture. The labeled sperm suspensions were immediately analyzed using flow cytometry with a BD Accuri™ C6 Plus Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ). Only sperm subpopulation negative for both FITC-PNA and PI (i.e., spermatozoa with intact acrosome and intact plasma membrane) was reported. Acquisitions of fluorescent data were achieved using BD Accuri C6 Plus software from at least 10,000 gated events collected.
Mitochondrial membrane potential (MMP)
The assessment of the MMP was done with a cell-permeant, red-orange fluorescent dye sequestered by active mitochondria: tetramethylrhodamine, ethyl ester (TMRE)53. In brief, 4 × 105 spermatozoa were incubated with 25 µM TMRE working solution. After 30 min, the spermatozoa were washed by centrifugation and analyzed by flow cytometry. At least 10,000 cells were examined for each analysis, and the percentage of spermatozoa with high MMP (i.e., TMRE stained cells) was reported.
Sperm oxidative stress
2,7-dichlorofluorescein diacetate (H2DCFDA) that de-esterifies in the presence of intracellular H2O2 to form a green fluorescent 2,7-dichlorofluorescein (DCF) was used to determine the level of oxidative stress in spermatozoa54. Briefly, the sperm suspension (2 × 106 cells/mL) was incubated with 20.5 µM H2DCFDA for 15 min. After the removal of excess unbound dye, PI (74.8 µM) was added to exclude the dead sperm population during flow cytometric analysis which at least 10,000 events per test were measured. In the present study, the percentage of viable spermatozoa having high intracellular ROS (i.e., DCF positive, PI negative cells) and intracellular ROS levels (mean DCF fluorescence intensity) per such cell were analyzed.
Histological examination of the hypothalamus, anterior pituitary gland, and testis
The hypothalamus, anterior pituitary, and one side of the testes harvested from each sacrificed mouse (3 mice/group for the hypothalamus and anterior pituitary and 5 mice/group for the testis) were prepared for histological examination using standard tissue preparation which involves four steps: (1) fixation with 10% neutral buffered formalin and dehydration with graded concentrations of ethyl alcohols, (2) paraffin embedding, (3) microtome sectioning (4–5 μm in thickness) and mounting onto appropriate glass microscope slides, and (4) staining with H&E. The processed slides were visualized for microscopic alteration using a Nikon ECLIPSE 80i light microscope (Nikon, Shinagawa, Tokyo, Japan).
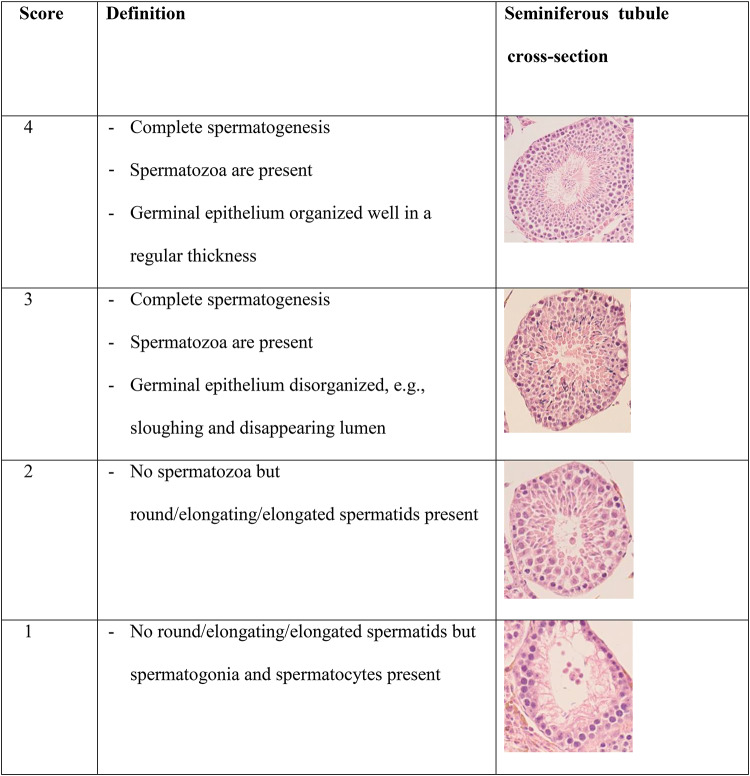
The histological alterations such as congestion, vacuolation, neuronal cell death, and abnormal astrocytes of the hypothalamus and anterior pituitary specimens were inspected in approximately 10 optical fields at ×200 (for hypothalamus) and ×400 (for anterior pituitary) microscopic magnifications using a qualitative, subjective approach. Testicular histological damage was assessed using Johnsen scoring system55,56, with some alterations. For each testis, at least 30 tubules in at least 10 different fields were observed at 400× magnification, and each tubule was given a score from 1 to 4 based on the presence of germ cell types, namely spermatogonia, spermatocytes, spermatids, and spermatozoa which the higher score indicates the better status of spermatogenesis. Histological classification of mouse seminiferous tubule cross-sections according to the modified Johnsen scoring system used in the present study is shown in Table 1.
Table 1.
Histological classification of mouse seminiferous tubule cross-sections according to the modified Johnsen scoring system.
Determination of tissue iron concentration by a chemical method
The other testis collected from each sacrificed mouse was stored separately at -80 °C in a clean, dry container until determining tissue iron content by a colorimetric method57. Upon analysis, the dried tissue was weighed and digested with acids. The non-heme iron present in the extract was examined by measuring optical density at 535 nm after adding chromogenic reagent (0.1% bathophenanthroline sulfonate and 1% thioglycolic acid). The tissue iron concentration (mg/g dried tissue) determined based on the iron standard curve was an average of duplicate samples.
Statistical analysis
Data are presented as mean ± standard error of the means (SEM). Statistical comparisons between normally distributed quantitative data were made using one-way analysis of variance (ANOVA) and the Tukey post hoc test. The Kruskal–Wallis test followed by the Bonferroni correction, to determine which groups differ from others, were performed to analyze semi-quantitative scores of the testes and non-normally distributed quantitative variables despite a log or square-root transformation. As not all variables were normally distributed, a statistical relationship between two quantitative variables was measured by the Spearman rank correlation coefficient. All statistical analyses were carried out using SPSS statistical program for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). Values of p < 0.05 were considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research project has been funded by Mahidol University (Fundamental Fund: fiscal year 2024 by National Science Research and Innovation Fund (NSRF); FF-173/2567).
Author contributions
SA, SR, CC, KV, PC, and KB performed the experiments, SA, SR, KN, KV, PC, and KB analyzed the data. PC, SS, and KB designed the experiments. PC, KV, and SS edited the manuscript, SS supervised the project. KB wrote, submitted, and revised the manuscript. All authors reviewed and approved the manuscript.
Data availability
All data related to this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bajwa, H., Basit, H. & Thalassemia. In StatPearls. Treasure Island (FL) (StatPearls Publishing, 2024).
- 2.Asadov, C. et al. β-Thalassemia intermedia: a comprehensive overview and novel approaches. Int. J. Hematol.108(1), 5–21. 10.1007/s12185-018-2411-9 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Modell, B. et al. Improved survival of Thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson.10, 1–8. 10.1186/1532-429X-10-42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaing, T. H. et al. Molecular genetics of β-thalassemia: a narrative review. Med. (Baltim).100(45), e27522. 10.1097/MD.0000000000027522 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, A. & Kan, Y. W. The prevention of Thalassemia. Cold Spring Harb Perspect. Med.3(2), a011775. 10.1101/cshperspect.a011775 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aessopos, A., Kati, M. & Farmakis, D. Heart disease in Thalassemia Intermedia: a review of the underlying pathophysiology. Haematologica92(5), 658–665. 10.3324/haematol.10915 (2007). [DOI] [PubMed] [Google Scholar]
- 7.De Sanctis, V. et al. Gonadal dysfunction in adult male patients with Thalassemia major: an update for clinicians caring for Thalassemia. Expert Rev. Hematol.10, 1095–1106. 10.1080/17474086.2017.1398080 (2017). [DOI] [PubMed] [Google Scholar]
- 8.De Sanctis, V. et al. Hypogonadism in male Thalassemia major patients: pathophysiology, diagnosis and treatment. Acta Biomed.89(2-S), 6–15. 10.23750/abm.v89i2-S.7082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Sanctis, V., Candini, G., Giovannini, M., Raiola, G. & Katz, M. Abnormal seminal parameters in patients with Thalassemia Intermedia and low serum folate levels. Pediatr. Endocrinol. Rev.8(Suppl 2), 310–313 (2011). [PubMed] [Google Scholar]
- 10.Li, L. et al. Ferroptosis is associated with oxygen-glucose deprivation/reoxygenation induced sertoli cell death. Int. J. Mol. Med.41, 3051–3062. 10.3892/ijmm.2018.3469 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Abarikwu, S. O., Wokoma, A. F. S., Mgbudom-Okah, C. J., Omeodu, S. I. & Ohanador, R. Effect of Fe and Cd co-exposure on testicular steroid metabolism, morphometry, and spermatogenesis in mice. Biol. Trace Elem. Res.190, 109–123. 10.1007/s12011-018-1536-2 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Perera, D. et al. Sperm DNA damage in potentially fertile homozygous beta-thalassaemia patients with iron overload. Hum. Reprod.17, 1820–1825. 10.1093/humrep/17.7.1820 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Liu, Y. et al. Effects of ferroptosis on male reproduction. Int. J. Mol. Sci.23(13), 7139. 10.3390/ijms23137139 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterston, R. H. et al. Initial sequencing and comparative analysis of the mouse genome. Nature420, 520–562. 10.1038/nature01262 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Brown, S. D. M. & Hancock, J. M. The mouse genome. In Vertebrate Genomes. (ed Volff, J-N.) 33–45 (Basel: Karger, 2006).
- 16.Morse, H. C. I. Building a better mouse: one hundred years of genetics and biology. In The Mouse in Biomedical Research. (ed Fox, J. G.) 1–11 (Elsevier, 2007).
- 17.Detloff, P. J. et al. Deletion and replacement of the mouse adult beta-globin genes by a plug and socket repeated targeting strategy. Mol. Cell. Biol.14(10), 6936–6943. https://doi.org/10.1128/MCB.14.10.6936 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang, B. et al. A mouse model for beta 0-thalassemia. Proc. Natl. Acad. Sci. U S A. 92(25), 11608–11612 (1995). 10.1073/pnas.92.25.11608 [DOI] [PMC free article] [PubMed]
- 19.McColl, B. & Vadolas, J. Animal models of β-hemoglobinopathies: utility and limitations. J. Blood Med.7, 263–274. 10.2147/JBM.S87955 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumfu, S., Fucharoen, S., Chattipakorn, S. C. & Chattipakorn, N. Cardiac complications in beta-thalassemia: from mice to men. Exp. Biol. Med. (Maywood). 242(11), 1126–1135. 10.1177/1535370217708977 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogiatzi, M. G. et al. Changes in bone microarchitecture and biomechanical properties in the th3 thalassemia mouse are associated with decreased bone turnover and occur during the period of bone accrual. Calcif Tissue Int.86(6), 484–494. 10.1007/s00223-010-9365-0 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ampel, N. M., Van Wyck, D. B., Aguirre, M. L., Willis, D. G. & Popp, R. A. Resistance to infection in murine beta-thalassemia. Infect. Immun.57(4), 1011–1017. 10.1128/iai.57.4.1011-1017.1989 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breda, L. et al. Therapeutic hemoglobin levels after gene transfer in β-thalassemia mice and in hematopoietic cells of β-thalassemia and sickle cells disease patients. PLoS One. 7(3), e32345. 10.1371/journal.pone.0032345 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao, S. H. et al. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril.89(5), 1183–1190. 10.1016/j.fertnstert.2007.05.029 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Agarwal, A. et al. in Oxidative Stress in Human Reproduction: Shedding Light on a Complicated Phenomenon. 190 (eds Agarwal, A.) (Springer Publishing, 2017).
- 26.Zalata, A. A., Ahmed, A. H., Allamaneni, S. S., Comhaire, F. H. & Agarwal, A. Relationship between acrosin activity of human spermatozoa and oxidative stress. Asian J. Androl.6, 313–318 (2004). [PubMed] [Google Scholar]
- 27.Tremellen, K. Oxidative stress and male infertility–a clinical perspective. Hum. Reprod. Update. 14(3), 243–258. 10.1093/humupd/dmn004 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Osadchuk, L. & Osadchuk, A. Genetic variability of spermatozoon production and morphology in laboratory mice. Bull. Exp. Biol. Med.149, 739–742. 10.1007/s10517-010-1040-y (2010). [DOI] [PubMed] [Google Scholar]
- 29.Sekine, N., Yokota, S. & Oshio, S. Sperm morphology is different in two common mouse strains. BPB Rep.4(5), 162–165 (2021). [Google Scholar]
- 30.Cross, C. E. et al. Oxygen radicals and human disease. Ann. Intern. Med.107(4), 526–545. 10.7326/0003-4819-107-4-526 (1987). [DOI] [PubMed] [Google Scholar]
- 31.Lucesoli, F., Caligiuri, M., Roberti, M. F., Perazzo, J. C. & Fraga, C. G. Dose dependent increase of oxidative damage in the testes of rats subjected to acute iron overload. Arch. Biochem. Biophys.372(1), 37–43. 10.1006/abbi.1999.1476 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Rossi, E. M. et al. Acute iron overload leads to hypothalamic-pituitary-gonadal axis abnormalities in female rats. Toxicol. Lett.240(1), 196–213. 10.1016/j.toxlet.2015.10.027 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Singer, S. T. et al. Fertility in transfusion-dependent thalassemia men: effects of iron burden on the reproductive axis. Am. J. Hematol.90, E190–E192. 10.1002/ajh.24083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, M. J. et al. Effect of iron overload on impaired fertility in male patients with transfusion-dependent beta-thalassemia. Pediatr. Res.83, 655–661. 10.1038/pr.2017.296 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Smith, L. B. & Walker, W. H. The regulation of spermatogenesis by Androgens. Semin Cell. Dev. Biol.30, 2–13. 10.1016/j.semcdb.2014.02.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grande, G. et al. The role of testosterone in spermatogenesis: lessons from proteome profiling of human spermatozoa in testosterone deficiency. Front. Endocrinol.13, 852661. 10.3389/fendo.2022.852661 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontogeorgos, G., Handy, S., Kovacs, K., Horvath, E. & Scheithauer, B. W. The anterior pituitary in hemochromatosis. Endocr. Pathol.7(2), 159–164. 10.1007/BF02739976 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Tvrda, E., Peer, R., Sikka, S. C. & Agarwal, A. Iron and copper in male reproduction: a double-edged sword. J. Assist. Reprod. Genet.32, 3–16. 10.1007/s10815-014-0344-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Osta, R., Grandpre, N., Monnin, N., Hubert, J. & Koscinski, I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic. Clin. Androl.27, 13. 10.1186/s12610-017-0057-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung, J. Y. et al. Styrene cytotoxicity in testicular leydig cells in vitro. Dev. Reprod.26, 99–105. 10.12717/DR.2022.26.3.99 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santambrogio, P. et al. Mitochondrial ferritin expression in adult mouse tissues. J. Histochem. Cytochem.55, 1129–1137. 10.1369/jhc.7A7273.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leichtmann-Bardoogo, Y. et al. Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload. Am. J. Physiol. Endocrinol. Metab.302, E1519–E1530. 10.1152/ajpendo.00007.2012 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Syamsunarno, M. R. A. A. et al. Short term iron overload injection alters reproduction organ and sperm quality in male mice. Adv. Anim. Vet. Sci.9(1), 35–41 (2021). [Google Scholar]
- 44.Sanyear, C. et al. Iron homeostasis in a mouse model of Thalassemia Intermedia is altered between adolescence and adulthood. PeerJ8, e8802. 10.7717/peerj.8802 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pholngam, N. et al. Cognitive impairment and hippocampal neuronal damage in β-thalassaemia mice. Sci. Rep.14, 10054. 10.1038/s41598-024-60459-y (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oudit, G. Y. et al. L–type Ca2þ channels provide a major pathway for iron entry into cardiomyocytes in iron–overload cardiomyopathy. Nat. Med.9, 1187–1194. 10.1038/nm920 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Buranaamnuay, K., Aiemongkot, S., Changsangfa, C. & Svasti, S. The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. Open. Vet. J.12(5), 602–611. 10.5455/OVJ.2022.v12.i5.2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brito, L. F. C. et al. Andrology laboratory review: evaluation of sperm concentration. Theriogenology85(9), 1507–1527. 10.1016/j.theriogenology.2016.01.002 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Buranaamnuay, K., Kettawan, A., Changsangfa, C. & Aiemongkot, S. Effect of chicken bone extract powder on epididymal sperm quality of male Wistar rats. Indonesian J. Anim. Vet. Sci.26(2), 74–81 (2021). [Google Scholar]
- 50.Correa, J. R. & Zavos, P. M. The hypoosmotic swelling test: its employment as an assay to evaluate the functional integrity of the frozen-thawed bovine sperm membrane. Theriogenology42(2), 351–360. 10.1016/0093-691x(94)90280-1 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Natali, I. et al. Scoring human sperm morphology using testsimplets and Diff-Quik slides. Fertil. Steril.99, 1227–1232e2. 10.1016/j.fertnstert.2012.11.047 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Nagy, S., Jansen, J., Topper, E. K. & Gadella, B. M. A triple-stain flow cytometric method to assess plasma- and acrosome-membrane integrity of cryopreserved bovine sperm immediately after thawing in presence of egg-yolk particles. Biol. Reprod.68(5), 1828–1835. 10.1095/biolreprod.102.011445 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Germain, N., Jouy, N., Marchetti, C. & Marchetti, P. Determination of mitochondrial membrane potential by flow cytometry in human sperm cells. In Manual of sperm function testing in human assisted reproduction (eds. Agarwal, A., Henkel, R. & Majzoub A). 58–71 (Cambridge University Press, 2021).
- 54.Gosalvez, J., Tvrda, E. & Agarwal, A. Free radical and superoxide reactivity detection in semen quality assessment: past, present, and future. J. Assist. Reprod. Genet.34, 697–707. 10.1007/s10815-017-0912-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnsen, S. G. Testicular biopsy score count- a method for registration of spermatogenesis in human normal values and results in 335 hypogonadal males. Hormones1, 2–25. 10.1159/000178170 (1970). [DOI] [PubMed] [Google Scholar]
- 56.Yalcin, T., Kaya, S., Tektemur, N. K. & Ozan, I. E. The methods used in histopathological evaluation of testis tissues. Batman Univ. J. Life Sci.10(1), 148–157 (2020). [Google Scholar]
- 57.Torrance, J. D. & Bothwell, T. H. A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr. J. Med. Sci.33(1), 9–11 (1968). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to this study are available from the corresponding author on reasonable request.