Abstract
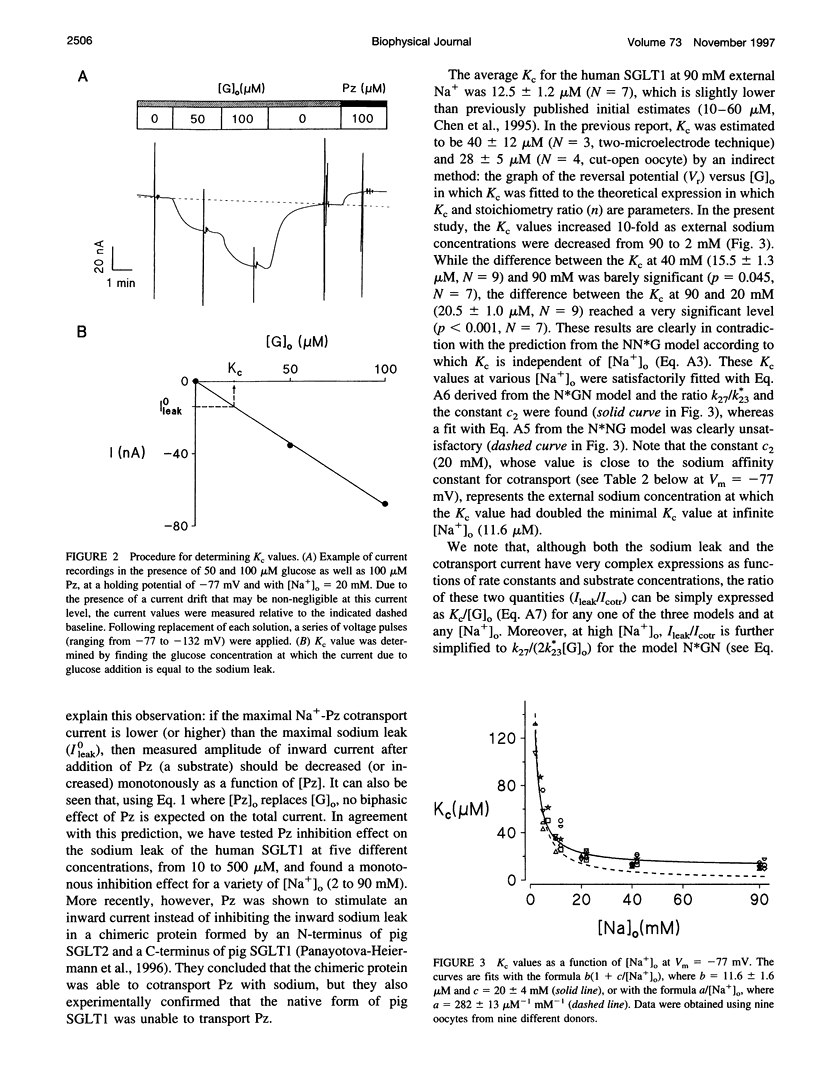
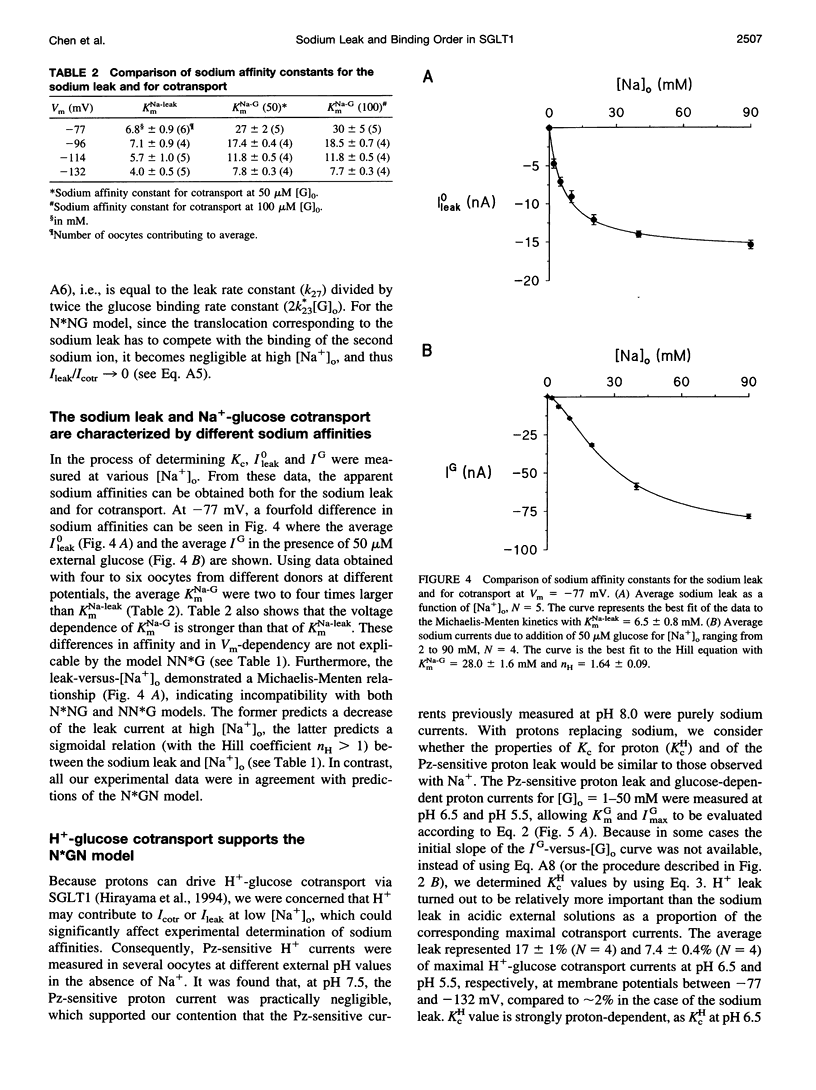
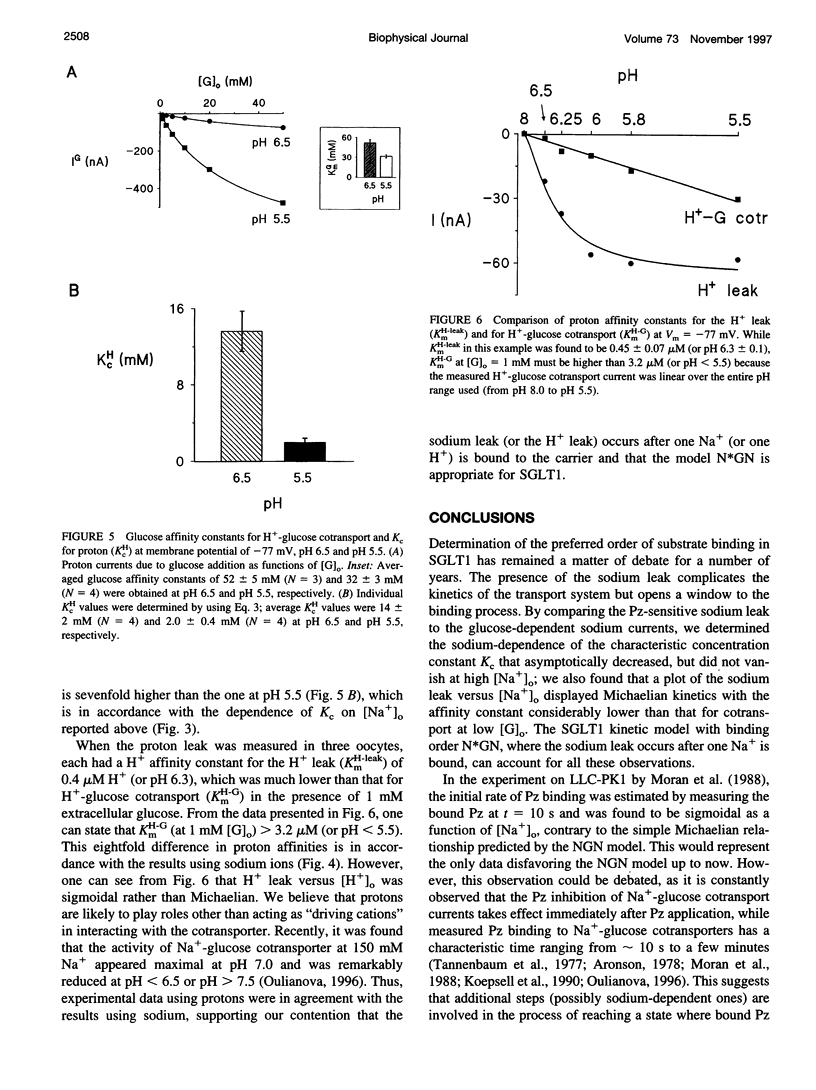
The Na+-glucose cotransporter (SGLT1) expressed in Xenopus laevis oocytes was shown to generate a phlorizin-sensitive sodium leak in the absence of sugars. Using the current model for SGLT1, where the sodium leak was presumed to occur after two sodium ions are bound to the free carrier before glucose binding, a characteristic concentration constant (Kc) was introduced to describe the relative importance of the sodium leak versus Na+-glucose cotransport currents. Kc represents the glucose concentration at which the Na+-glucose cotransport current is equal to the sodium leak. As both the sodium leak and the Na+-glucose cotransport current are predicted to occur after the binding of two sodium ions, the model predicted that Kc should be sodium-independent. However, by using a two-microelectrode voltage-clamp technique, the observed Kc was shown to depend strongly on the external sodium concentration ([Na+]o): it was four times higher at 5 mM [Na+]o than at 20 mM [Na+]o. In addition, the magnitude of the sodium leak varied as a function of [Na+]o in a Michaelian fashion, and the sodium affinity constant for the sodium leak was 2-4 times lower than that for cotransport in the presence of low external glucose concentrations (50 or 100 microM), whereas the current model predicted a sigmoidal sodium dependence of the sodium leak and identical sodium affinities for the sodium leak and the Na+-glucose cotransport. These observations indicate that the sodium leak occurs after one sodium ion is associated with the carrier and agree with predictions from a model with the binding order sodium-glucose-sodium. This conclusion was also supported by experiments performed where protons replaced Na+ as a "driving cation."
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol. 1978 Jul 21;42(1):81–98. doi: 10.1007/BF01870395. [DOI] [PubMed] [Google Scholar]
- Bennett E., Kimmich G. A. Na+ binding to the Na(+)-glucose cotransporter is potential dependent. Am J Physiol. 1992 Feb;262(2 Pt 1):C510–C516. doi: 10.1152/ajpcell.1992.262.2.C510. [DOI] [PubMed] [Google Scholar]
- Bennett E., Kimmich G. A. The molecular mechanism and potential dependence of the Na+/glucose cotransporter. Biophys J. 1996 Apr;70(4):1676–1688. doi: 10.1016/S0006-3495(96)79730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. Z., Coady M. J., Jackson F., Berteloot A., Lapointe J. Y. Thermodynamic determination of the Na+: glucose coupling ratio for the human SGLT1 cotransporter. Biophys J. 1995 Dec;69(6):2405–2414. doi: 10.1016/S0006-3495(95)80110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. Z., Coady M. J., Lapointe J. Y. Fast voltage clamp discloses a new component of presteady-state currents from the Na(+)-glucose cotransporter. Biophys J. 1996 Nov;71(5):2544–2552. doi: 10.1016/S0006-3495(96)79447-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu C., Berteloot A. Allosterism and Na(+)-D-glucose cotransport kinetics in rabbit jejunal vesicles: compatibility with mixed positive and negative cooperativities in a homo- dimeric or tetrameric structure and experimental evidence for only one transport protein involved. J Membr Biol. 1993 Mar;132(2):95–113. doi: 10.1007/BF00239000. [DOI] [PubMed] [Google Scholar]
- Coady M. J., Pajor A. M., Wright E. M. Sequence homologies among intestinal and renal Na+/glucose cotransporters. Am J Physiol. 1990 Oct;259(4 Pt 1):C605–C610. doi: 10.1152/ajpcell.1990.259.4.C605. [DOI] [PubMed] [Google Scholar]
- Crane R. K. The gradient hypothesis and other models of carrier-mediated active transport. Rev Physiol Biochem Pharmacol. 1977;78:99–159. doi: 10.1007/BFb0027722. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Turk E., Wright E. M. Homology of the human intestinal Na+/glucose and Escherichia coli Na+/proline cotransporters. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5748–5752. doi: 10.1073/pnas.86.15.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama B. A., Loo D. D., Wright E. M. Protons drive sugar transport through the Na+/glucose cotransporter (SGLT1). J Biol Chem. 1994 Aug 26;269(34):21407–21410. [PubMed] [Google Scholar]
- Kessler M., Semenza G. The small-intestinal Na+, D-glucose cotransporter: an asymmetric gated channel (or pore) responsive to delta psi. J Membr Biol. 1983;76(1):27–56. doi: 10.1007/BF01871452. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A. Membrane potentials and the mechanism of intestinal Na(+)-dependent sugar transport. J Membr Biol. 1990 Mar;114(1):1–27. doi: 10.1007/BF01869381. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. Na+-coupled sugar transport: membrane potential-dependent Km and Ki for Na+. Am J Physiol. 1988 Oct;255(4 Pt 1):C486–C494. doi: 10.1152/ajpcell.1988.255.4.C486. [DOI] [PubMed] [Google Scholar]
- Koepsell H., Fritzsch G., Korn K., Madrala A. Two substrate sites in the renal Na(+)-D-glucose cotransporter studied by model analysis of phlorizin binding and D-glucose transport measurements. J Membr Biol. 1990 Mar;114(2):113–132. doi: 10.1007/BF01869093. [DOI] [PubMed] [Google Scholar]
- Lee W. S., Kanai Y., Wells R. G., Hediger M. A. The high affinity Na+/glucose cotransporter. Re-evaluation of function and distribution of expression. J Biol Chem. 1994 Apr 22;269(16):12032–12039. [PubMed] [Google Scholar]
- Loo D. D., Hazama A., Supplisson S., Turk E., Wright E. M. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5767–5771. doi: 10.1073/pnas.90.12.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lostao M. P., Hirayama B. A., Loo D. D., Wright E. M. Phenylglucosides and the Na+/glucose cotransporter (SGLT1): analysis of interactions. J Membr Biol. 1994 Nov;142(2):161–170. doi: 10.1007/BF00234938. [DOI] [PubMed] [Google Scholar]
- Mackenzie B., Panayotova-Heiermann M., Loo D. D., Lever J. E., Wright E. M. SAAT1 is a low affinity Na+/glucose cotransporter and not an amino acid transporter. A reinterpretation. J Biol Chem. 1994 Sep 9;269(36):22488–22491. [PubMed] [Google Scholar]
- Moran A., Davis L. J., Turner R. J. High affinity phlorizin binding to the LLC-PK1 cells exhibits a sodium:phlorizin stoichiometry of 2:1. J Biol Chem. 1988 Jan 5;263(1):187–192. [PubMed] [Google Scholar]
- Ohta T., Isselbacher K. J., Rhoads D. B. Regulation of glucose transporters in LLC-PK1 cells: effects of D-glucose and monosaccharides. Mol Cell Biol. 1990 Dec;10(12):6491–6499. doi: 10.1128/mcb.10.12.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotova-Heiermann M., Loo D. D., Kong C. T., Lever J. E., Wright E. M. Sugar binding to Na+/glucose cotransporters is determined by the carboxyl-terminal half of the protein. J Biol Chem. 1996 Apr 26;271(17):10029–10034. doi: 10.1074/jbc.271.17.10029. [DOI] [PubMed] [Google Scholar]
- Panayotova-Heiermann M., Loo D. D., Wright E. M. Kinetics of steady-state currents and charge movements associated with the rat Na+/glucose cotransporter. J Biol Chem. 1995 Nov 10;270(45):27099–27105. doi: 10.1074/jbc.270.45.27099. [DOI] [PubMed] [Google Scholar]
- Parent L., Supplisson S., Loo D. D., Wright E. M. Electrogenic properties of the cloned Na+/glucose cotransporter: I. Voltage-clamp studies. J Membr Biol. 1992 Jan;125(1):49–62. doi: 10.1007/BF00235797. [DOI] [PubMed] [Google Scholar]
- Parent L., Supplisson S., Loo D. D., Wright E. M. Electrogenic properties of the cloned Na+/glucose cotransporter: II. A transport model under nonrapid equilibrium conditions. J Membr Biol. 1992 Jan;125(1):63–79. doi: 10.1007/BF00235798. [DOI] [PubMed] [Google Scholar]
- Quick M. W., Naeve J., Davidson N., Lester H. A. Incubation with horse serum increases viability and decreases background neurotransmitter uptake in Xenopus oocytes. Biotechniques. 1992 Sep;13(3):357–361. [PubMed] [Google Scholar]
- RIKLIS E., QUASTEL J. H. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can J Biochem Physiol. 1958 Mar;36(3):347–362. [PubMed] [Google Scholar]
- Restrepo D., Kimmich G. A. Kinetic analysis of mechanism of intestinal Na+-dependent sugar transport. Am J Physiol. 1985 May;248(5 Pt 1):C498–C509. doi: 10.1152/ajpcell.1985.248.5.C498. [DOI] [PubMed] [Google Scholar]
- Restrepo D., Kimmich G. A. Phlorizin binding to isolated enterocytes: membrane potential and sodium dependence. J Membr Biol. 1986;89(3):269–280. doi: 10.1007/BF01870669. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Semenza G., Kessler M., Hosang M., Weber J., Schmidt U. Biochemistry of the Na+, D-glucose cotransporter of the small-intestinal brush-border membrane. The state of the art in 1984. Biochim Biophys Acta. 1984 Sep 3;779(3):343–379. doi: 10.1016/0304-4157(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C., Toggenburger G., Kessler M., Rothstein A., Semenza G. High-affinity phlorizin binding to brush border membranes from small intestine: identity with (a part of) the glucose transport system, dependence on Na +-gradient, partial purification. J Supramol Struct. 1977;6(4):519–533. doi: 10.1002/jss.400060406. [DOI] [PubMed] [Google Scholar]
- Tarpey P. S., Shirazi-Beechey S. P., Beechey R. B. Molecular characterisation of the Na+/glucose co-transporter from the sheep parotid gland acinar cell. Biochem Soc Trans. 1994 Aug;22(3):264S–264S. doi: 10.1042/bst022264s. [DOI] [PubMed] [Google Scholar]
- Toggenburger G., Kessler M., Semenza G. Phlorizin as a probe of the small-intestinal Na+,D-glucose cotransporter. A model. Biochim Biophys Acta. 1982 Jun 14;688(2):557–571. doi: 10.1016/0005-2736(82)90367-4. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol. 1982;70(1):37–45. doi: 10.1007/BF01871587. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Stoichiometric studies of the renal outer cortical brush border membrane D-glucose transporter. J Membr Biol. 1982;67(1):73–80. doi: 10.1007/BF01868649. [DOI] [PubMed] [Google Scholar]
- Umbach J. A., Coady M. J., Wright E. M. Intestinal Na+/glucose cotransporter expressed in Xenopus oocytes is electrogenic. Biophys J. 1990 Jun;57(6):1217–1224. doi: 10.1016/S0006-3495(90)82640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyhl M., Spangenberg J., Püschel B., Poppe R., Dekel C., Fritzsch G., Haase W., Koepsell H. Cloning of a membrane-associated protein which modifies activity and properties of the Na(+)-D-glucose cotransporter. J Biol Chem. 1993 Nov 25;268(33):25041–25053. [PubMed] [Google Scholar]
- Wells R. G., Pajor A. M., Kanai Y., Turk E., Wright E. M., Hediger M. A. Cloning of a human kidney cDNA with similarity to the sodium-glucose cotransporter. Am J Physiol. 1992 Sep;263(3 Pt 2):F459–F465. doi: 10.1152/ajprenal.1992.263.3.F459. [DOI] [PubMed] [Google Scholar]
- Wright E. M. The intestinal Na+/glucose cotransporter. Annu Rev Physiol. 1993;55:575–589. doi: 10.1146/annurev.ph.55.030193.003043. [DOI] [PubMed] [Google Scholar]


