Abstract
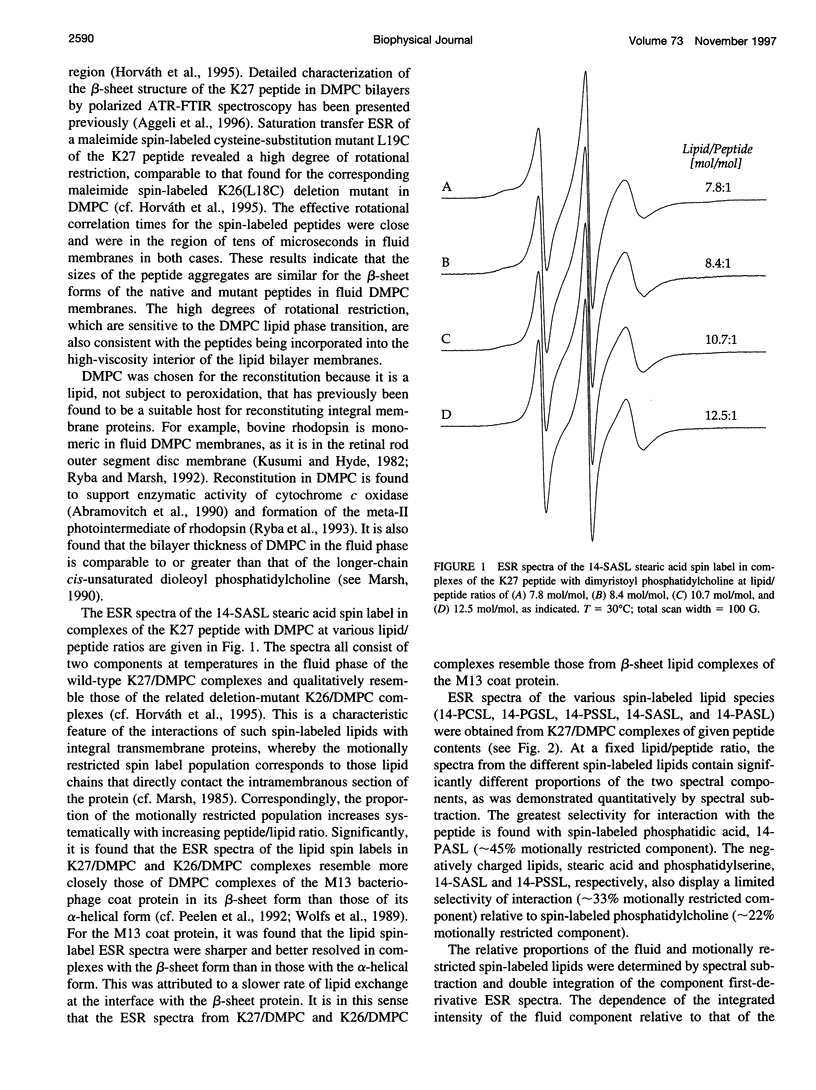
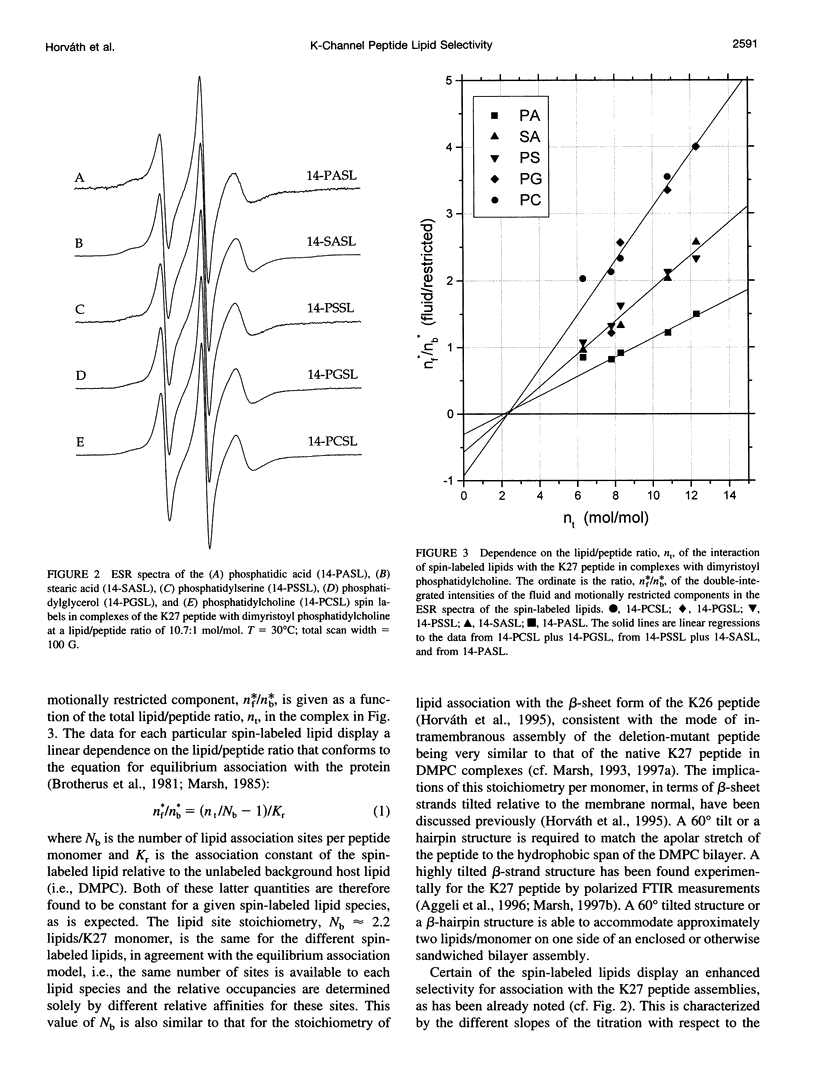
Lipid-peptide interactions with the 27-residue peptide of sequence KLEALYILMVLGFFGFFTLGIMLSYIR reconstituted as beta-sheet assemblies in dimyristoylphosphatidylcholine bilayers have been studied by electron spin resonance (ESR) spectroscopy with spin-labeled lipids. The peptide corresponds to residues 42-68 of the IsK voltage-gated K+ channel protein and contains the single putative transmembrane span of this protein. Lipid-peptide interactions give rise to a second component in the ESR spectra of lipids spin-labeled on the 14C atom of the chain that corresponds to restriction of the lipid mobility by direct interaction with the peptide assemblies. From the dependence on the lipid/peptide ratio, the stoichiometry of lipid interaction is found to be about two phospholipids/peptide monomer. The sequence of selectivity for lipid association with the peptide assemblies is in the order phosphatidic acid > stearic acid = phosphatidylserine > phosphatidylglycerol = phosphatidylcholine. Comparison with previous data for a corresponding 26-residue mutant peptide with a single deletion of the apolar residue Leu2 (Horvath et al., 1995. Biochemistry 34:3893-3898), indicates a very similar mode of membrane incorporation for native and mutant peptides, but a strongly modified pattern and degree of specificity for the interaction with negatively charged lipids. The latter is interpreted in terms of the relative orientations of the charged amino acid side chains in the beta-sheet assemblies of the native and deletion-mutant peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitch D. A., Marsh D., Powell G. L. Activation of beef-heart cytochrome c oxidase by cardiolipin and analogues of cardiolipin. Biochim Biophys Acta. 1990 Oct 24;1020(1):34–42. doi: 10.1016/0005-2728(90)90090-q. [DOI] [PubMed] [Google Scholar]
- Aggeli A., Boden N., Cheng Y. L., Findlay J. B., Knowles P. F., Kovatchev P., Turnbull P. J. Peptides modeled on the transmembrane region of the slow voltage-gated IsK potassium channel: structural characterization of peptide assemblies in the beta-strand conformation. Biochemistry. 1996 Dec 17;35(50):16213–16221. doi: 10.1021/bi960891g. [DOI] [PubMed] [Google Scholar]
- Attali B., Guillemare E., Lesage F., Honoré E., Romey G., Lazdunski M., Barhanin J. The protein IsK is a dual activator of K+ and Cl- channels. Nature. 1993 Oct 28;365(6449):850–852. doi: 10.1038/365850a0. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim I., Bach D., Shai Y. Spectroscopic and functional characterization of the putative transmembrane segment of the minK potassium channel. Biochemistry. 1993 Mar 9;32(9):2371–2377. doi: 10.1021/bi00060a031. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim I., Strahilevitz J., Bach D., Shai Y. Secondary structure and membrane localization of synthetic segments and a truncated form of the IsK (minK) protein. Biochemistry. 1994 Jun 7;33(22):6966–6973. doi: 10.1021/bi00188a028. [DOI] [PubMed] [Google Scholar]
- Bogusz S., Boxer A., Busath D. D. An SS1-SS2 beta-barrel structure for the voltage-activated potassium channel. Protein Eng. 1992 Jun;5(4):285–293. doi: 10.1093/protein/5.4.285. [DOI] [PubMed] [Google Scholar]
- Brophy J. Association of proteolipid apoproteins from bovine myelin with phospholipid in bilayer vesicles. FEBS Lett. 1977 Dec 1;84(1):92–96. doi: 10.1016/0014-5793(77)81064-8. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Griffith O. H., Brotherus M. O., Jost P. C., Silvius J. R., Hokin L. E. Lipid--protein multiple binding equilibria in membranes. Biochemistry. 1981 Sep 1;20(18):5261–5267. doi: 10.1021/bi00521a026. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Cornea R. L., Jones L. R., Autry J. M., Thomas D. D. Mutation and phosphorylation change the oligomeric structure of phospholamban in lipid bilayers. Biochemistry. 1997 Mar 11;36(10):2960–2967. doi: 10.1021/bi961955q. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Esmann M., Marsh D. Spin-label studies on the origin of the specificity of lipid-protein interactions in Na+,K+-ATPase membranes from Squalus acanthias. Biochemistry. 1985 Jul 2;24(14):3572–3578. doi: 10.1021/bi00335a027. [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Miller C. Site-specific mutations in a minimal voltage-dependent K+ channel alter ion selectivity and open-channel block. Neuron. 1991 Sep;7(3):403–408. doi: 10.1016/0896-6273(91)90292-8. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci. 1990 Jun;13(6):201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- Honoré E., Attali B., Romey G., Heurteaux C., Ricard P., Lesage F., Lazdunski M., Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991 Oct;10(10):2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth L. I., Brophy P. J., Marsh D. Influence of lipid headgroup on the specificity and exchange dynamics in lipid-protein interactions. A spin-label study of myelin proteolipid apoprotein-phospholipid complexes. Biochemistry. 1988 Jul 12;27(14):5296–5304. doi: 10.1021/bi00414a052. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Heimburg T., Kovachev P., Findlay J. B., Hideg K., Marsh D. Integration of a K+ channel-associated peptide in a lipid bilayer: conformation, lipid-protein interactions, and rotational diffusion. Biochemistry. 1995 Mar 28;34(12):3893–3898. doi: 10.1021/bi00012a004. [DOI] [PubMed] [Google Scholar]
- King D. S., Fields C. G., Fields G. B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int J Pept Protein Res. 1990 Sep;36(3):255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Kusumi A., Hyde J. S. Spin-label saturation-transfer electron spin resonance detection of transient association of rhodopsin in reconstituted membranes. Biochemistry. 1982 Nov 9;21(23):5978–5983. doi: 10.1021/bi00266a039. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsh D. Dichroic ratios in polarized Fourier transform infrared for nonaxial symmetry of beta-sheet structures. Biophys J. 1997 Jun;72(6):2710–2718. doi: 10.1016/S0006-3495(97)78914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Peptide models for membrane channels. Biochem J. 1996 Apr 15;315(Pt 2):345–361. doi: 10.1042/bj3150345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T., Kakizuka A., Takumi T., Ohkubo H., Nakanishi S. Molecular cloning and sequence analysis of human genomic DNA encoding a novel membrane protein which exhibits a slowly activating potassium channel activity. Biochem Biophys Res Commun. 1989 May 30;161(1):176–181. doi: 10.1016/0006-291x(89)91577-5. [DOI] [PubMed] [Google Scholar]
- Peelen S. J., Sanders J. C., Hemminga M. A., Marsh D. Stoichiometry, selectivity, and exchange dynamics of lipid-protein interaction with bacteriophage M13 coat protein studied by spin label electron spin resonance. Effects of protein secondary structure. Biochemistry. 1992 Mar 17;31(10):2670–2677. doi: 10.1021/bi00125a006. [DOI] [PubMed] [Google Scholar]
- Ryba N. J., Marsh D. Protein rotational diffusion and lipid/protein interactions in recombinants of bovine rhodopsin with saturated diacylphosphatidylcholines of different chain lengths studied by conventional and saturation-transfer electron spin resonance. Biochemistry. 1992 Aug 25;31(33):7511–7518. doi: 10.1021/bi00148a011. [DOI] [PubMed] [Google Scholar]
- Ryba N. J., Marsh D., Uhl R. The kinetics and thermodynamics of bleaching of rhodopsin in dimyristoylphosphatidylcholine. Identification of meta-I, meta-II, and meta-III intermediates. Biophys J. 1993 Jun;64(6):1801–1812. doi: 10.1016/S0006-3495(93)81551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt R. B., Hemminga M. A. The in situ aggregational and conformational state of the major coat protein of bacteriophage M13 in phospholipid bilayers mimicking the inner membrane of host Escherichia coli. Biochemistry. 1991 Nov 19;30(46):11147–11154. doi: 10.1021/bi00110a018. [DOI] [PubMed] [Google Scholar]
- Spruijt R. B., Wolfs C. J., Hemminga M. A. Aggregation-related conformational change of the membrane-associated coat protein of bacteriophage M13. Biochemistry. 1989 Nov 14;28(23):9158–9165. doi: 10.1021/bi00449a030. [DOI] [PubMed] [Google Scholar]
- Takumi T., Moriyoshi K., Aramori I., Ishii T., Oiki S., Okada Y., Ohkubo H., Nakanishi S. Alteration of channel activities and gating by mutations of slow ISK potassium channel. J Biol Chem. 1991 Nov 25;266(33):22192–22198. [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T., Maylie J., Adelman J. P. Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys J. 1995 Sep;69(3):904–908. doi: 10.1016/S0006-3495(95)79964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs J. A., Horváth L. I., Marsh D., Watts A., Hemminga M. A. Spin-label ESR of bacteriophage M13 coat protein in mixed lipid bilayers. Characterization of molecular selectivity of charged phospholipids for the bacteriophage M13 coat protein in lipid bilayers. Biochemistry. 1989 Dec 26;28(26):9995–10001. doi: 10.1021/bi00452a018. [DOI] [PubMed] [Google Scholar]