Abstract
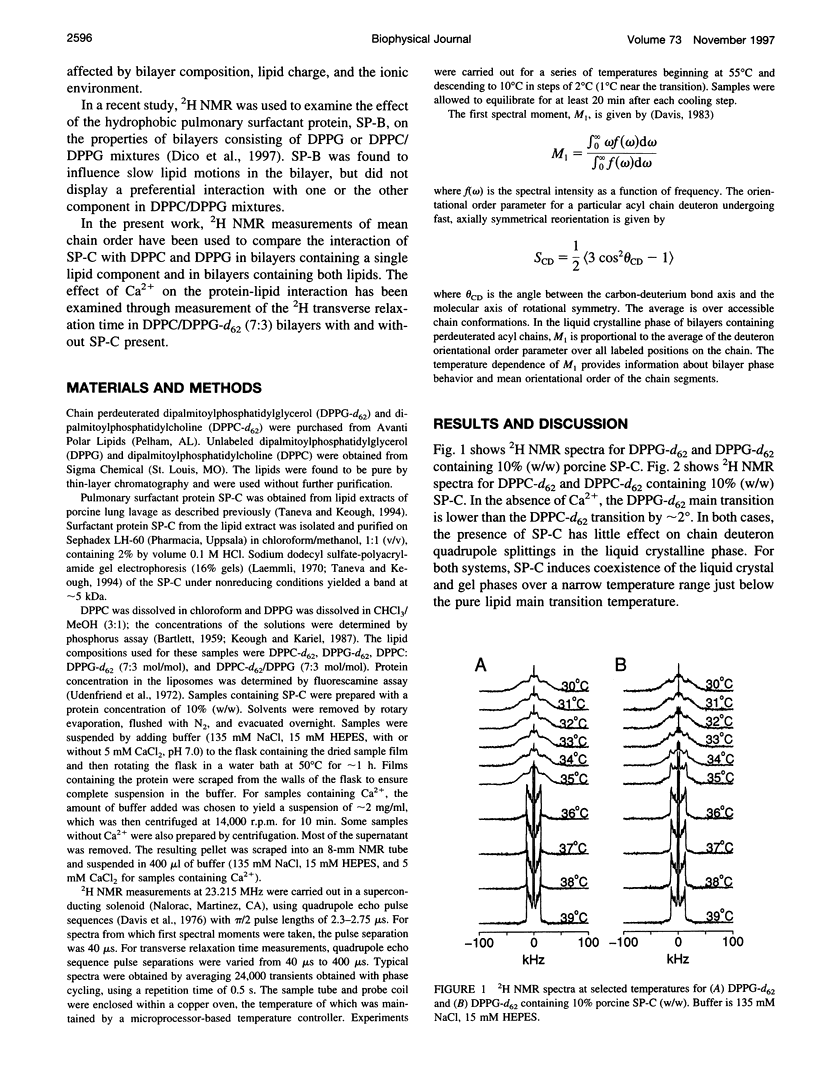
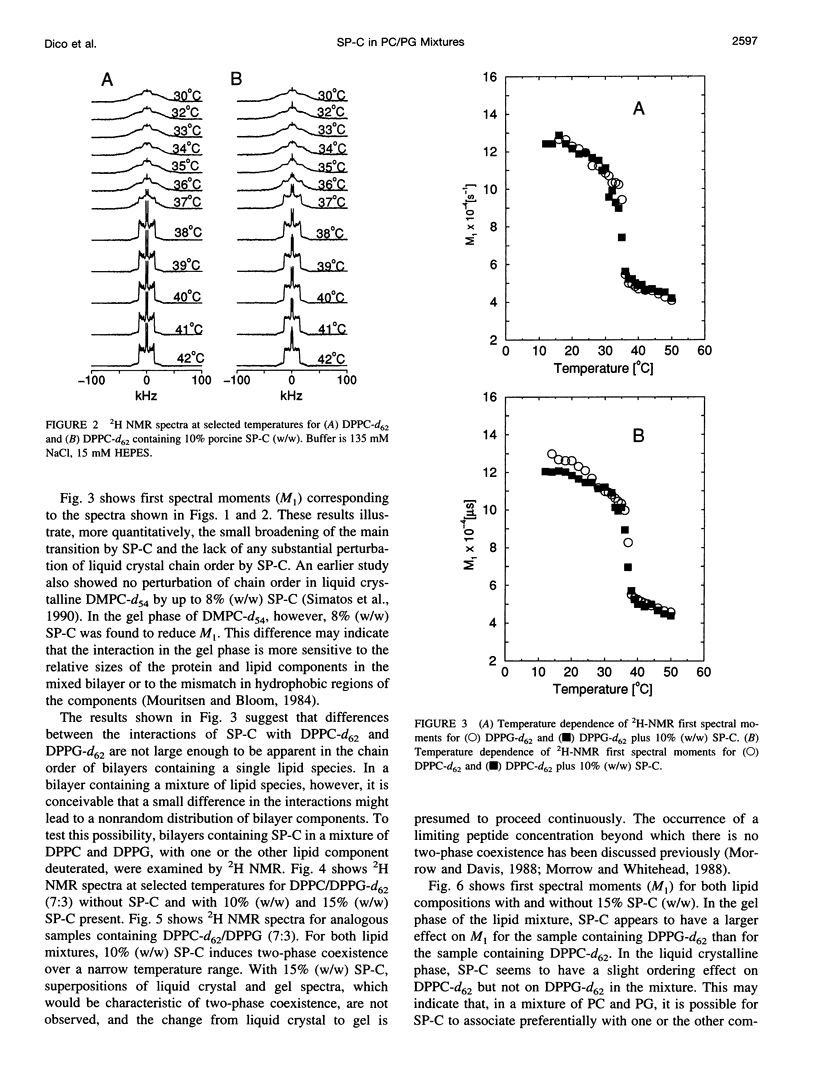
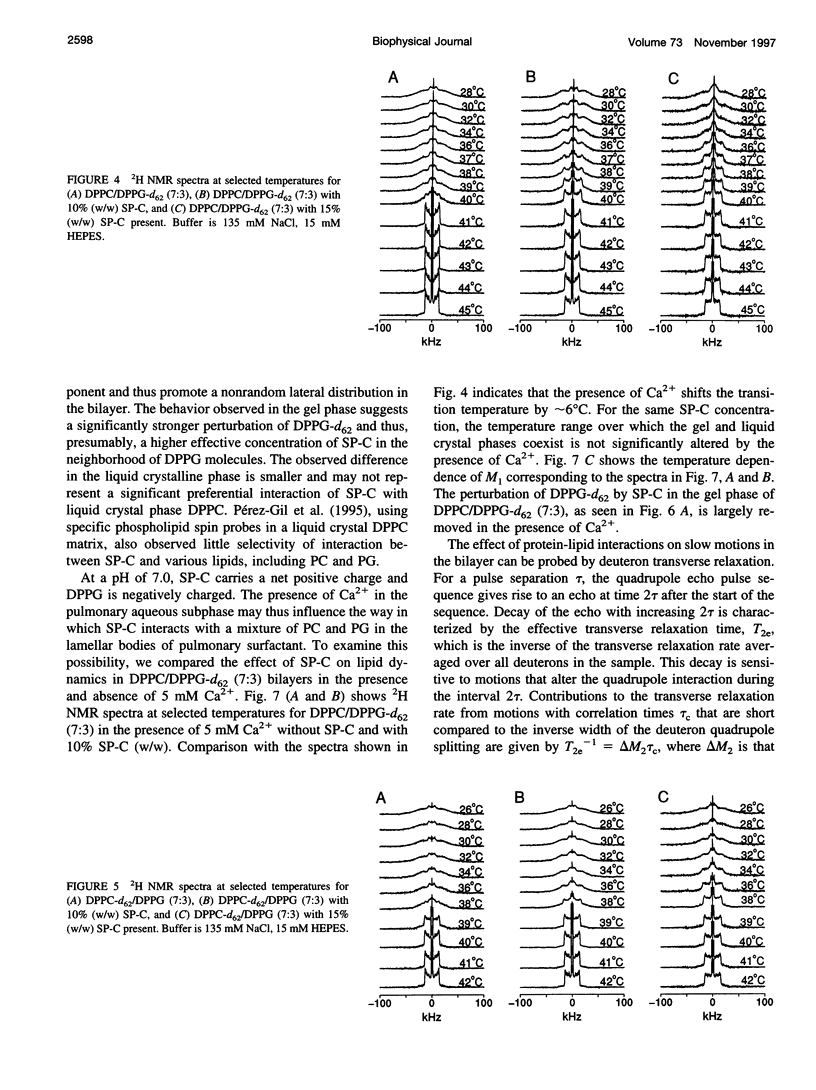
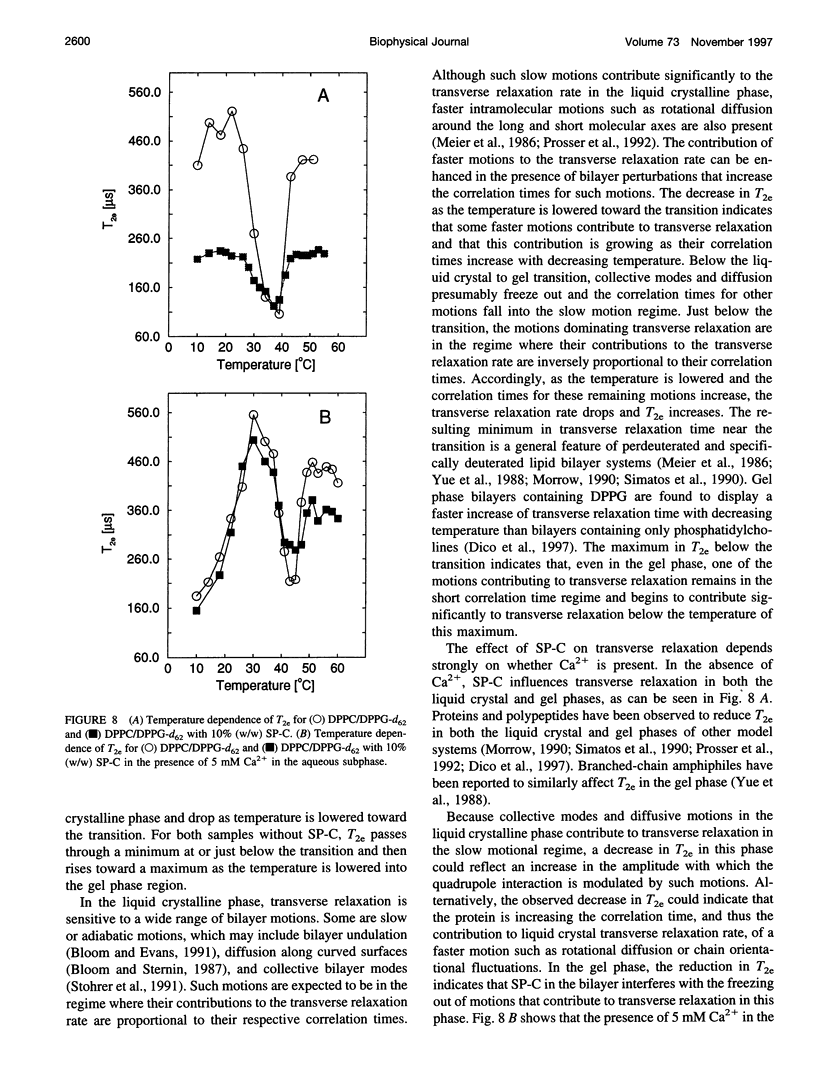
Porcine pulmonary surfactant-associated protein SP-C was incorporated into bilayers of chain-perdeuterated dipalmitoylphosphatidylglycerol (DPPG-d62) and chain-perdeuterated dipalmitoyl-phosphatidylcholine (DPPC-d62) and into bilayers containing 70 mol% dipalmitoyl-phosphatidylcholine (DPPC) and 30 mol% DPPG-d62 or 70 mol% DPPC-d62 and 30 mol% dipalmitoylphosphatidylglycerol (DPPG). The effect of SP-C on the phase behavior, lipid chain order, and dynamics in these bilayers was examined by using deuterium nuclear magnetic resonance. SP-C was found to have a similar effect on the chain order and phase behavior of DPPC-d62 and DPPG-d62 in bilayers with a single lipid component. In gel phase DPPC/DPPG (7:3) bilayers with one or the other lipid component chain-perdeuterated, SP-C was found to affect first spectral moment more strongly for DPPG-d62 than for DPPC-d62. This may indicate that SP-C induced a nonrandom lateral distribution in the mixed lipid bilayer. SP-C was also found to influence motions responsible for deuteron transverse relaxation in both the gel and liquid crystalline phases. The presence of 5 mM Ca2+ in the aqueous phase substantially altered the effect of SP-C on transverse relaxation in the bilayer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Dico A. S., Hancock J., Morrow M. R., Stewart J., Harris S., Keough K. M. Pulmonary surfactant protein SP-B interacts similarly with dipalmitoylphosphatidylglycerol and dipalmitoylphosphatidylcholine in phosphatidylcholine/phosphatidylglycerol mixtures. Biochemistry. 1997 Apr 8;36(14):4172–4177. doi: 10.1021/bi962693v. [DOI] [PubMed] [Google Scholar]
- Johansson J., Curstedt T., Robertson B. The proteins of the surfactant system. Eur Respir J. 1994 Feb;7(2):372–391. doi: 10.1183/09031936.94.07020372. [DOI] [PubMed] [Google Scholar]
- Kahn M. C., Anderson G. J., Anyan W. R., Hall S. B. Phosphatidylcholine molecular species of calf lung surfactant. Am J Physiol. 1995 Nov;269(5 Pt 1):L567–L573. doi: 10.1152/ajplung.1995.269.5.L567. [DOI] [PubMed] [Google Scholar]
- Keough K. M., Kariel N. Differential scanning calorimetric studies of aqueous dispersions of phosphatidylcholines containing two polyenoic chains. Biochim Biophys Acta. 1987 Aug 7;902(1):11–18. doi: 10.1016/0005-2736(87)90130-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Davis J. H. Differential scanning calorimetry and 2H NMR studies of the phase behavior of gramicidin-phosphatidylcholine mixtures. Biochemistry. 1988 Mar 22;27(6):2024–2032. doi: 10.1021/bi00406a032. [DOI] [PubMed] [Google Scholar]
- Morrow M. R. Transverse nuclear spin relaxation in phosphatidylcholine bilayers containing gramicidin. Biochim Biophys Acta. 1990 Apr 13;1023(2):197–205. doi: 10.1016/0005-2736(90)90414-j. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Whitehead J. P. A phenomenological model for lipid-protein bilayers with critical mixing. Biochim Biophys Acta. 1988 Jun 22;941(2):271–277. doi: 10.1016/0005-2736(88)90188-5. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984 Aug;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterlaken-Dijksterhuis M. A., Haagsman H. P., van Golde L. M., Demel R. A. Interaction of lipid vesicles with monomolecular layers containing lung surfactant proteins SP-B or SP-C. Biochemistry. 1991 Aug 20;30(33):8276–8281. doi: 10.1021/bi00247a024. [DOI] [PubMed] [Google Scholar]
- Pastrana B., Mautone A. J., Mendelsohn R. Fourier transform infrared studies of secondary structure and orientation of pulmonary surfactant SP-C and its effect on the dynamic surface properties of phospholipids. Biochemistry. 1991 Oct 15;30(41):10058–10064. doi: 10.1021/bi00105a033. [DOI] [PubMed] [Google Scholar]
- Pauls K. P., MacKay A. L., Söderman O., Bloom M., Tanjea A. K., Hodges R. S. Dynamic properties of the backbone of an integral membrane polypeptide measured by 2H-NMR. Eur Biophys J. 1985;12(1):1–11. doi: 10.1007/BF00254089. [DOI] [PubMed] [Google Scholar]
- Prosser R. S., Davis J. H., Mayer C., Weisz K., Kothe G. Deuterium NMR relaxation studies of peptide-lipid interactions. Biochemistry. 1992 Oct 6;31(39):9355–9363. doi: 10.1021/bi00154a005. [DOI] [PubMed] [Google Scholar]
- Pérez-Gil J., Casals C., Marsh D. Interactions of hydrophobic lung surfactant proteins SP-B and SP-C with dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylglycerol bilayers studied by electron spin resonance spectroscopy. Biochemistry. 1995 Mar 28;34(12):3964–3971. doi: 10.1021/bi00012a014. [DOI] [PubMed] [Google Scholar]
- Pérez-Gil J., Tucker J., Simatos G., Keough K. M. Interfacial adsorption of simple lipid mixtures combined with hydrophobic surfactant protein from pig lung. Biochem Cell Biol. 1992 May;70(5):332–338. doi: 10.1139/o92-051. [DOI] [PubMed] [Google Scholar]
- Simatos G. A., Forward K. B., Morrow M. R., Keough K. M. Interaction between perdeuterated dimyristoylphosphatidylcholine and low molecular weight pulmonary surfactant protein SP-C. Biochemistry. 1990 Jun 19;29(24):5807–5814. doi: 10.1021/bi00476a023. [DOI] [PubMed] [Google Scholar]
- Taneva S. G., Keough K. M. Dynamic surface properties of pulmonary surfactant proteins SP-B and SP-C and their mixtures with dipalmitoylphosphatidylcholine. Biochemistry. 1994 Dec 13;33(49):14660–14670. doi: 10.1021/bi00253a003. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Vandenbussche G., Clercx A., Curstedt T., Johansson J., Jörnvall H., Ruysschaert J. M. Structure and orientation of the surfactant-associated protein C in a lipid bilayer. Eur J Biochem. 1992 Jan 15;203(1-2):201–209. doi: 10.1111/j.1432-1033.1992.tb19848.x. [DOI] [PubMed] [Google Scholar]
- Weaver T. E., Whitsett J. A. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem J. 1991 Jan 15;273(Pt 2):249–264. doi: 10.1042/bj2730249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J., Thewalt J. L., Cushley R. J. Deuterium nuclear magnetic resonance study of the interaction of branched chain compounds (phytanic acid, phytol) with a phospholipid model membrane. Chem Phys Lipids. 1988 Dec;49(3):205–213. doi: 10.1016/0009-3084(88)90008-4. [DOI] [PubMed] [Google Scholar]


