Abstract
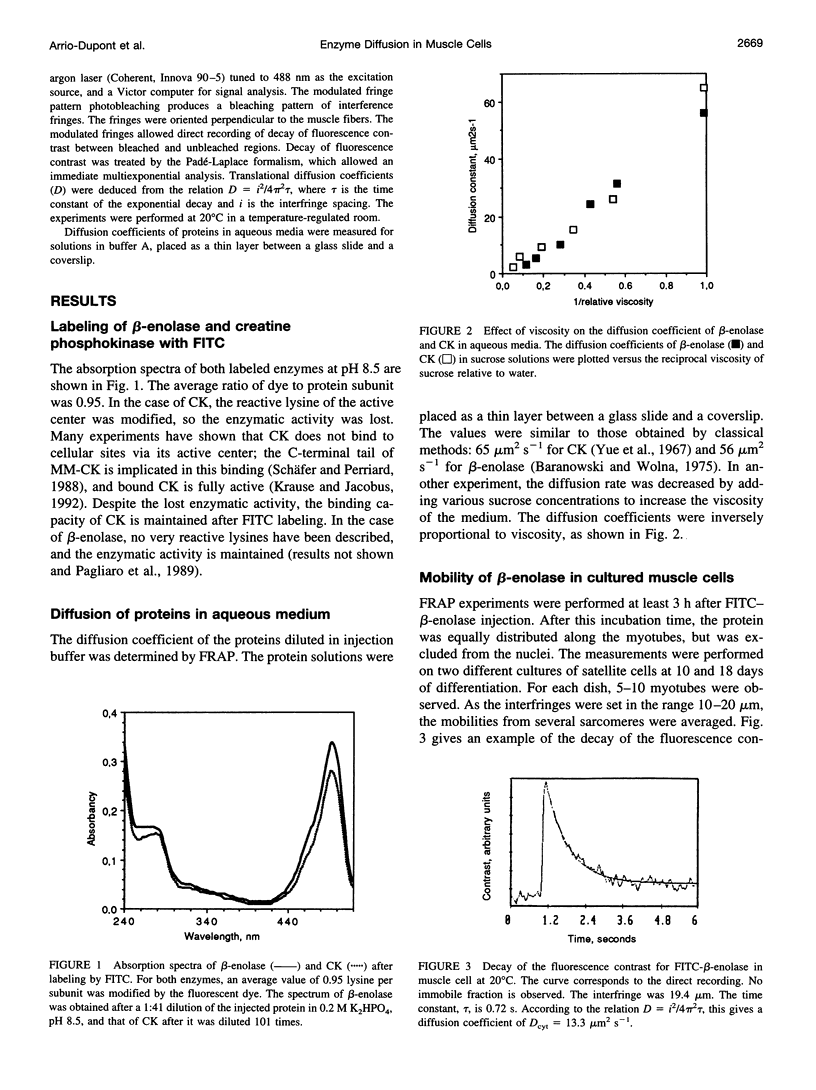
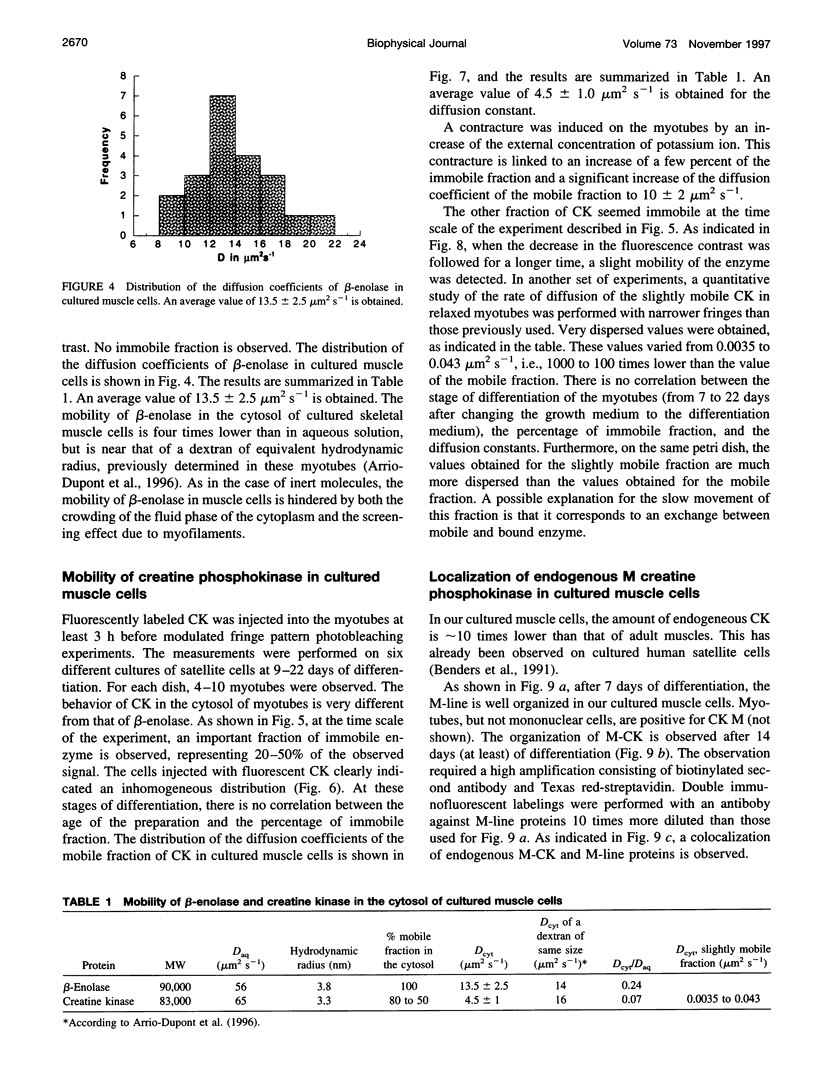
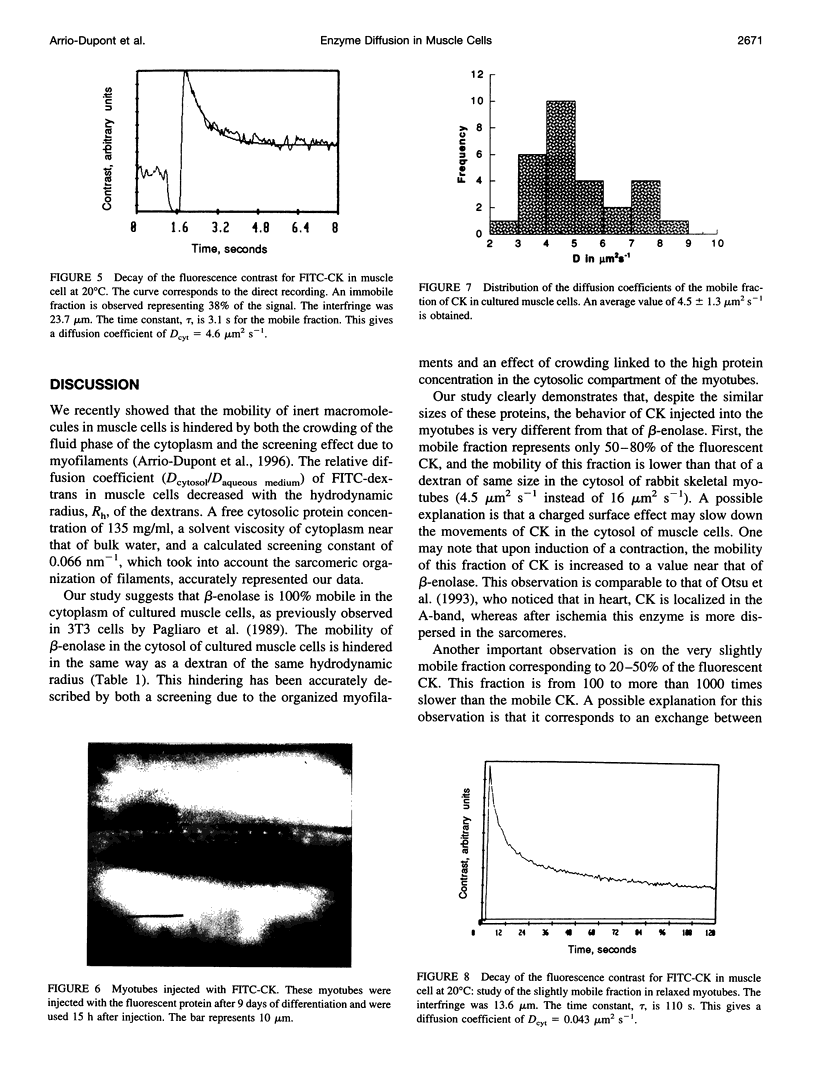
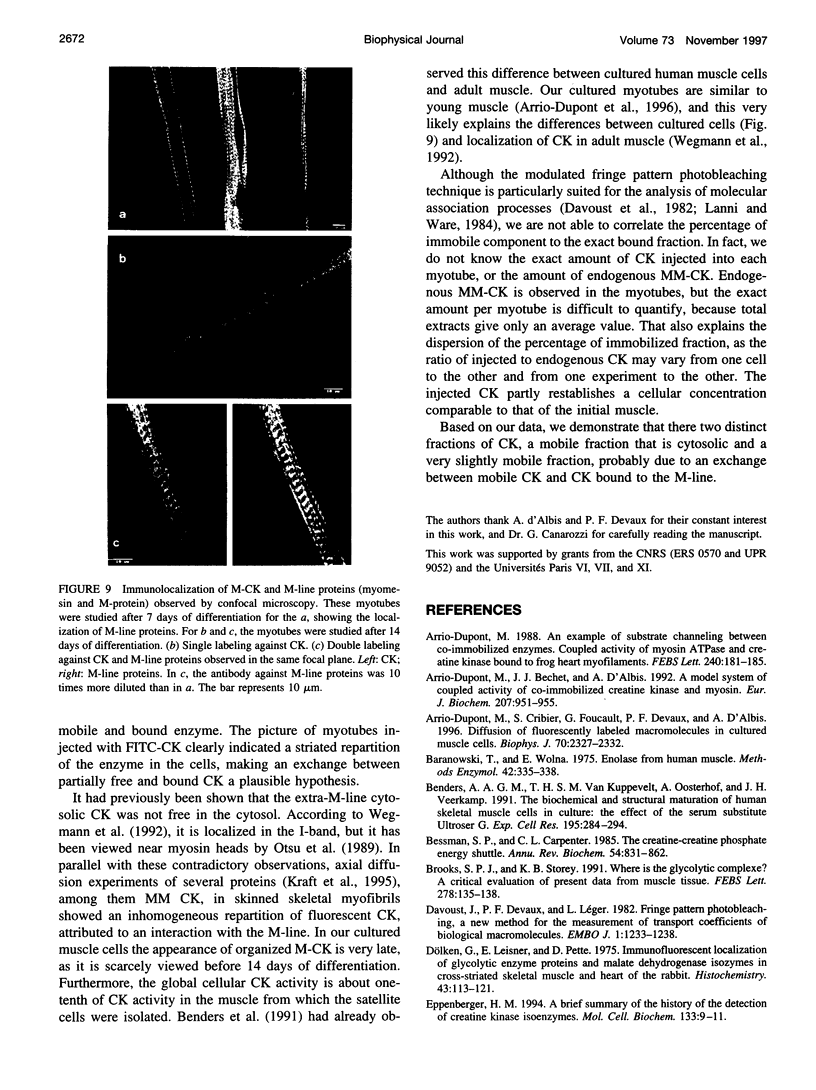
The diffusion of beta-enolase and creatine phosphokinase in muscle cells has been studied by modulated fringe pattern photobleaching. Beta-enolase is mobile in the sarcoplasm. At 20 degrees C, the diffusion coefficient is 13.5 +/- 2.5 microm2 s(-1) in the cytosol and 56 microm2 s(-1) in aqueous media. As in the case of dextrans of the same hydrodynamic radius, its mobility is hindered by both the crowding of the fluid phase of the cytoplasm and the screening effect due to myofilaments. A fraction of creatine phosphokinase is mobile in the sarcoplasm. Its diffusion coefficient in the cytosol, 4.5 +/- 1 microm2 s(-1), is lower than that of the dextran of equivalent size. The other fraction (20 to 50%) is very slightly mobile, with an apparent diffusion coefficient varying from 0.0035 to 0.043 microm2 s(-1). This low mobility might be attributed to exchange between free and bound creatine phosphokinase. The bound fraction of the endogenous enzyme was localized by immunocytofluorescence on the cultured muscle cells. Our results favor a localization of bound cytosolic creatine phosphokinase on the M-line and a diffuse distribution in all myotubes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrio-Dupont M. An example of substrate channeling between co-immobilized enzymes. Coupled activity of myosin ATPase and creatine kinase bound to frog heart myofilaments. FEBS Lett. 1988 Nov 21;240(1-2):181–185. doi: 10.1016/0014-5793(88)80364-8. [DOI] [PubMed] [Google Scholar]
- Arrio-Dupont M., Béchet J. J., d'Albis A. A model system of coupled activity of co-immobilized creatine kinase and myosin. Eur J Biochem. 1992 Aug 1;207(3):951–955. doi: 10.1111/j.1432-1033.1992.tb17129.x. [DOI] [PubMed] [Google Scholar]
- Arrio-Dupont M., Cribier S., Foucault G., Devaux P. F., d'Albis A. Diffusion of fluorescently labeled macromolecules in cultured muscle cells. Biophys J. 1996 May;70(5):2327–2332. doi: 10.1016/S0006-3495(96)79798-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski T., Wolna E. Enolase from human muscle. Methods Enzymol. 1975;42:335–338. doi: 10.1016/0076-6879(75)42137-1. [DOI] [PubMed] [Google Scholar]
- Benders A. A., van Kuppevelt T. H., Oosterhof A., Veerkamp J. H. The biochemical and structural maturation of human skeletal muscle cells in culture: the effect of the serum substitute Ultroser G. Exp Cell Res. 1991 Aug;195(2):284–294. doi: 10.1016/0014-4827(91)90375-5. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Carpenter C. L. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Brooks S. P., Storey K. B. Where is the glycolytic complex? A critical evaluation of present data from muscle tissue. FEBS Lett. 1991 Jan 28;278(2):135–138. doi: 10.1016/0014-5793(91)80101-8. [DOI] [PubMed] [Google Scholar]
- Davoust J., Devaux P. F., Leger L. Fringe pattern photobleaching, a new method for the measurement of transport coefficients of biological macromolecules. EMBO J. 1982;1(10):1233–1238. doi: 10.1002/j.1460-2075.1982.tb00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölken G., Leisner E., Pette D. Immunofluorescent localization of glycogenolytic and glycolytic enzyme proteins and of malate dehydrogenase isozymes in cross-striated skeletal muscle and heart of the rabbit. Histochemistry. 1975;43(2):113–121. doi: 10.1007/BF00492440. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M. A brief summary of the history of the detection of creatine kinase isoenzymes. Mol Cell Biochem. 1994 Apr-May;133-134:9–11. doi: 10.1007/BF01267944. [DOI] [PubMed] [Google Scholar]
- Fritz-Wolf K., Schnyder T., Wallimann T., Kabsch W. Structure of mitochondrial creatine kinase. Nature. 1996 May 23;381(6580):341–345. doi: 10.1038/381341a0. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- Kenyon G. L., Reed G. H. Creatine kinase: structure-activity relationships. Adv Enzymol Relat Areas Mol Biol. 1983;54:367–426. doi: 10.1002/9780470122990.ch6. [DOI] [PubMed] [Google Scholar]
- Knull H. R., Walsh J. L. Association of glycolytic enzymes with the cytoskeleton. Curr Top Cell Regul. 1992;33:15–30. doi: 10.1016/b978-0-12-152833-1.50007-1. [DOI] [PubMed] [Google Scholar]
- Kraft T., Messerli M., Rothen-Rutishauser B., Perriard J. C., Wallimann T., Brenner B. Equilibration and exchange of fluorescently labeled molecules in skinned skeletal muscle fibers visualized by confocal microscopy. Biophys J. 1995 Oct;69(4):1246–1258. doi: 10.1016/S0006-3495(95)80018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S. M., Jacobus W. E. Specific enhancement of the cardiac myofibrillar ATPase by bound creatine kinase. J Biol Chem. 1992 Feb 5;267(4):2480–2486. [PubMed] [Google Scholar]
- Labeit S., Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995 Oct 13;270(5234):293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Lanni F., Ware B. R. Detection and characterization of actin monomers, oligomers, and filaments in solution by measurement of fluorescence photobleaching recovery. Biophys J. 1984 Jul;46(1):97–110. doi: 10.1016/S0006-3495(84)84002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K. Physical properties of cytoplasm. Curr Opin Cell Biol. 1994 Feb;6(1):3–9. doi: 10.1016/0955-0674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Maughan D., Lord C. Protein diffusivities in skinned frog skeletal muscle fibers. Adv Exp Med Biol. 1988;226:75–84. [PubMed] [Google Scholar]
- Maughan D., Wegner E. On the organization and diffusion of glycolytic enzymes in skeletal muscle. Prog Clin Biol Res. 1989;315:137–147. [PubMed] [Google Scholar]
- Merkulova T., Lucas M., Jabet C., Lamandé N., Rouzeau J. D., Gros F., Lazar M., Keller A. Biochemical characterization of the mouse muscle-specific enolase: developmental changes in electrophoretic variants and selective binding to other proteins. Biochem J. 1997 May 1;323(Pt 3):791–800. doi: 10.1042/bj3230791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrot G., Cribier S., Devaux P. F., Geldwerth D., Davoust J., Bureau J. F., Fellmann P., Herve P., Frilley B. Asymmetric lateral mobility of phospholipids in the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6863–6867. doi: 10.1073/pnas.83.18.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann W. M., Gautel M., Steiner F., van der Ven P. F., Weber K., Fürst D. O. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J Cell Biol. 1996 Sep;134(6):1441–1453. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu N., Hirata M., Tuboi S., Miyazawa K. Immunocytochemical localization of creatine kinase M in canine myocardial cells: most creatine kinase M is distributed in the A-band. J Histochem Cytochem. 1989 Oct;37(10):1465–1470. doi: 10.1177/37.10.2778305. [DOI] [PubMed] [Google Scholar]
- Otsu N., Yamaguchi I., Komatsu E., Miyazawa K. Changes in creatine kinase M localization in acute ischemic myocardial cells. Immunoelectron microscopic studies. Circ Res. 1993 Nov;73(5):935–942. doi: 10.1161/01.res.73.5.935. [DOI] [PubMed] [Google Scholar]
- Pagliaro L., Kerr K., Taylor D. L. Enolase exists in the fluid phase of cytoplasm in 3T3 cells. J Cell Sci. 1989 Oct;94(Pt 2):333–342. doi: 10.1242/jcs.94.2.333. [DOI] [PubMed] [Google Scholar]
- Rider C. C., Taylor C. B. Enolase isoenzymes in rat tissues. Electrophoretic, chromatographic, immunological and kinetic properties. Biochim Biophys Acta. 1974 Sep 13;365(1):285–300. doi: 10.1016/0005-2795(74)90273-6. [DOI] [PubMed] [Google Scholar]
- Schäfer B. W., Perriard J. C. Intracellular targeting of isoproteins in muscle cytoarchitecture. J Cell Biol. 1988 Apr;106(4):1161–1170. doi: 10.1083/jcb.106.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh S., Mukunda K., Katiyar S. S. Evidence for proximal cysteine and lysine residues present at the nucleotide domain of rabbit muscle creatine kinase. Biochim Biophys Acta. 1993 Dec 8;1203(2):276–281. doi: 10.1016/0167-4838(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Small J. V., Fürst D. O., Thornell L. E. The cytoskeletal lattice of muscle cells. Eur J Biochem. 1992 Sep 15;208(3):559–572. doi: 10.1111/j.1432-1033.1992.tb17220.x. [DOI] [PubMed] [Google Scholar]
- Sosa H., Popp D., Ouyang G., Huxley H. E. Ultrastructure of skeletal muscle fibers studied by a plunge quick freezing method: myofilament lengths. Biophys J. 1994 Jul;67(1):283–292. doi: 10.1016/S0006-3495(94)80479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere P. A., Ovadi J. Enzyme-enzyme interactions and their metabolic role. FEBS Lett. 1990 Aug 1;268(2):360–364. doi: 10.1016/0014-5793(90)81286-w. [DOI] [PubMed] [Google Scholar]
- Trinick J. Titin and nebulin: protein rulers in muscle? Trends Biochem Sci. 1994 Oct;19(10):405–409. doi: 10.1016/0968-0004(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Eppenberger H. M. Localization and function of M-line-bound creatine kinase. M-band model and creatine phosphate shuttle. Cell Muscle Motil. 1985;6:239–285. doi: 10.1007/978-1-4757-4723-2_8. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H. M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992 Jan 1;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann G., Zanolla E., Eppenberger H. M., Wallimann T. In situ compartmentation of creatine kinase in intact sarcomeric muscle: the acto-myosin overlap zone as a molecular sieve. J Muscle Res Cell Motil. 1992 Aug;13(4):420–435. doi: 10.1007/BF01738037. [DOI] [PubMed] [Google Scholar]
- Welch G. R. On the role of organized multienzyme systems in cellular metabolism: a general synthesis. Prog Biophys Mol Biol. 1977;32(2):103–191. [PubMed] [Google Scholar]
- Yue R. H., Palmieri R. H., Olson O. E., Kuby S. A. Studies on adenosine triphosphate transphophorylases. V. Studies on the polypeptide chains of the crystalline adenosine triphosphate-creatine transphosphorylase from rabbit skeletal muscle. Biochemistry. 1967 Oct;6(10):3204–3227. doi: 10.1021/bi00862a031. [DOI] [PubMed] [Google Scholar]