Abstract
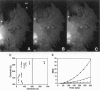
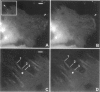
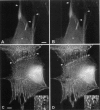
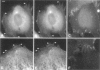
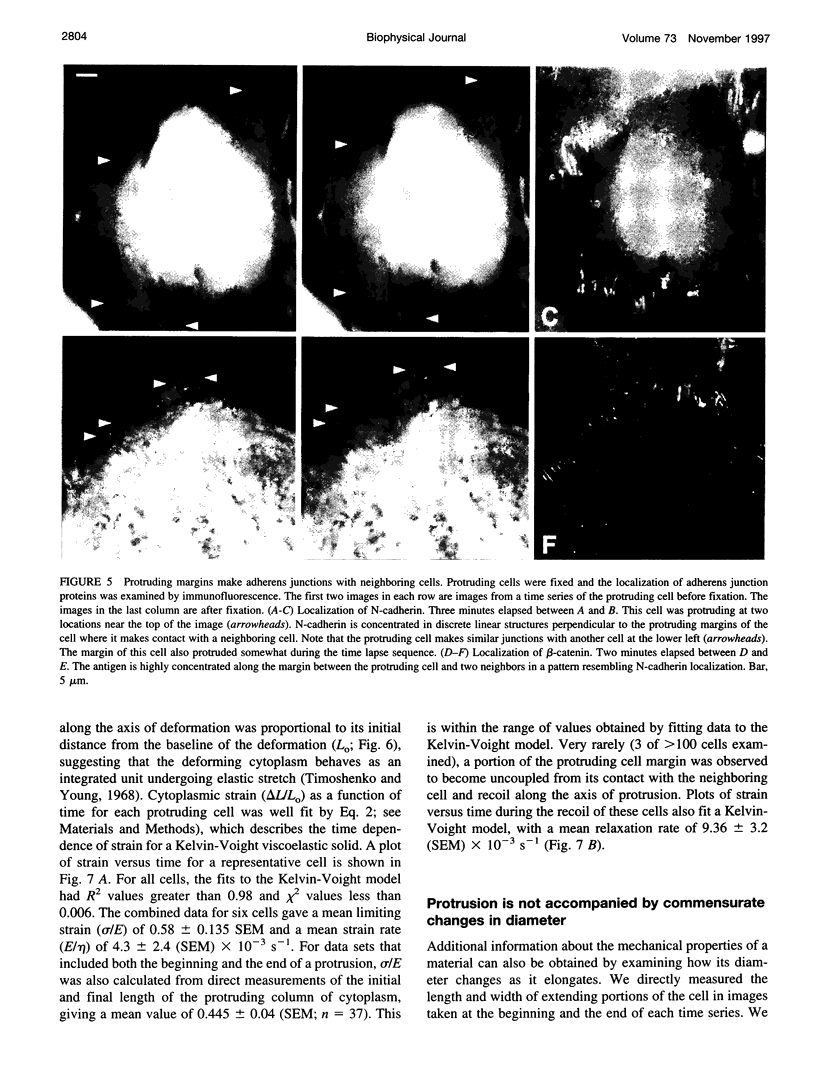
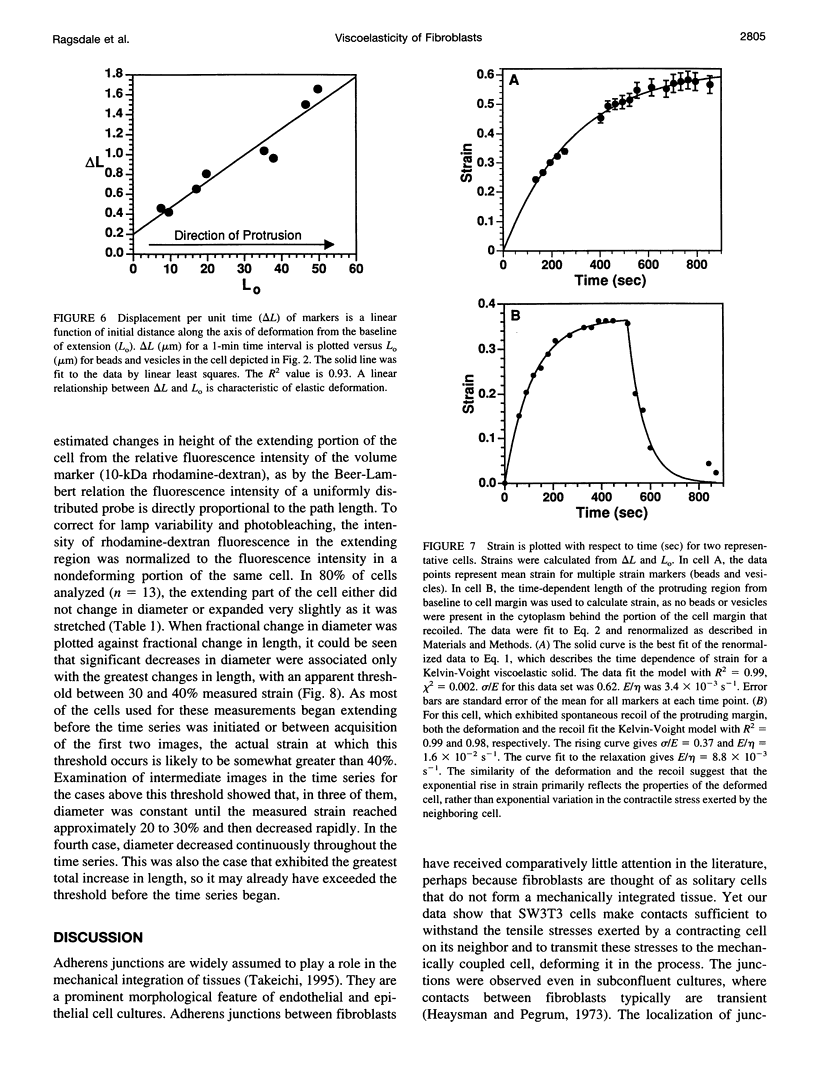
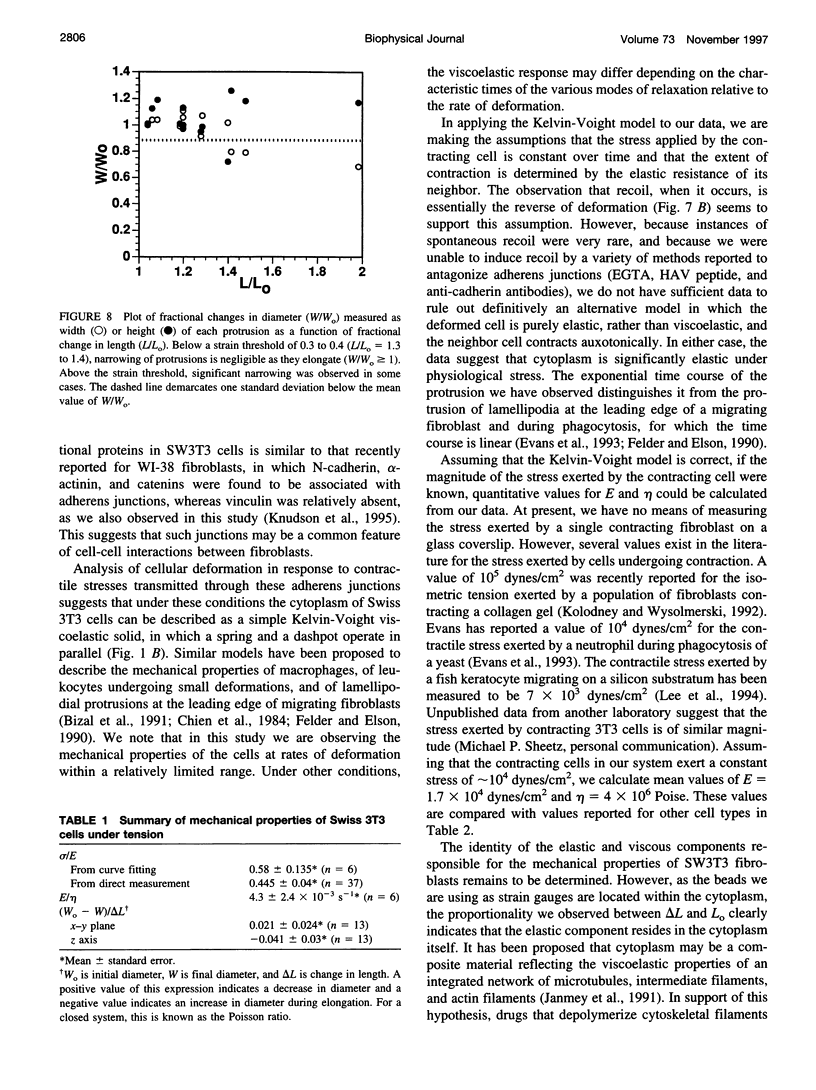
Cytoplasmic deformation was monitored by observing the displacements of 200-nm green fluorescent beads microinjected into the cytoplasm of Swiss 3T3 fibroblasts. We noted a novel protrusion of nonruffling cell margins that was accompanied by axial flow of beads and cytoplasmic vesicles as far as 50 microm behind the protruding plasma membrane. Fluorescent analog cytochemistry and immunofluorescence localization of F-actin, alpha-actinin, N-cadherin, and beta-catenin showed that the protruding margins of deforming cells were mechanically coupled to neighboring cells by adherens junctions. Observations suggested that protrusion resulted from passive linear deformation in response to tensile stress exerted by centripetal contraction of the neighboring cell. The time dependence of cytoplasmic strain calculated from the displacements of beads and vesicles was fit quantitatively by a Kelvin-Voight model for a viscoelastic solid with a mean limiting strain of 0.58 and a mean strain rate of 4.3 x 10(-3) s(-1). In rare instances, the deforming cell and its neighbor spontaneously became uncoupled, and recoil of the protruding margin was observed. The time dependence of strain during recoil also fit a Kelvin-Voight model with similar parameters, suggesting that the kinetics of deformation primarily reflect the mechanical properties of the deformed cell rather than the contractile properties of its neighbor. The existence of mechanical coupling between adjacent fibroblasts through adherens junctions and the viscoelastic responses of cells to tension transmitted directly from cell to cell are factors that must be taken into account to fully understand the role of fibroblasts in such biological processes as wound closure and extracellular matrix remodeling during tissue development.
Full text
PDF
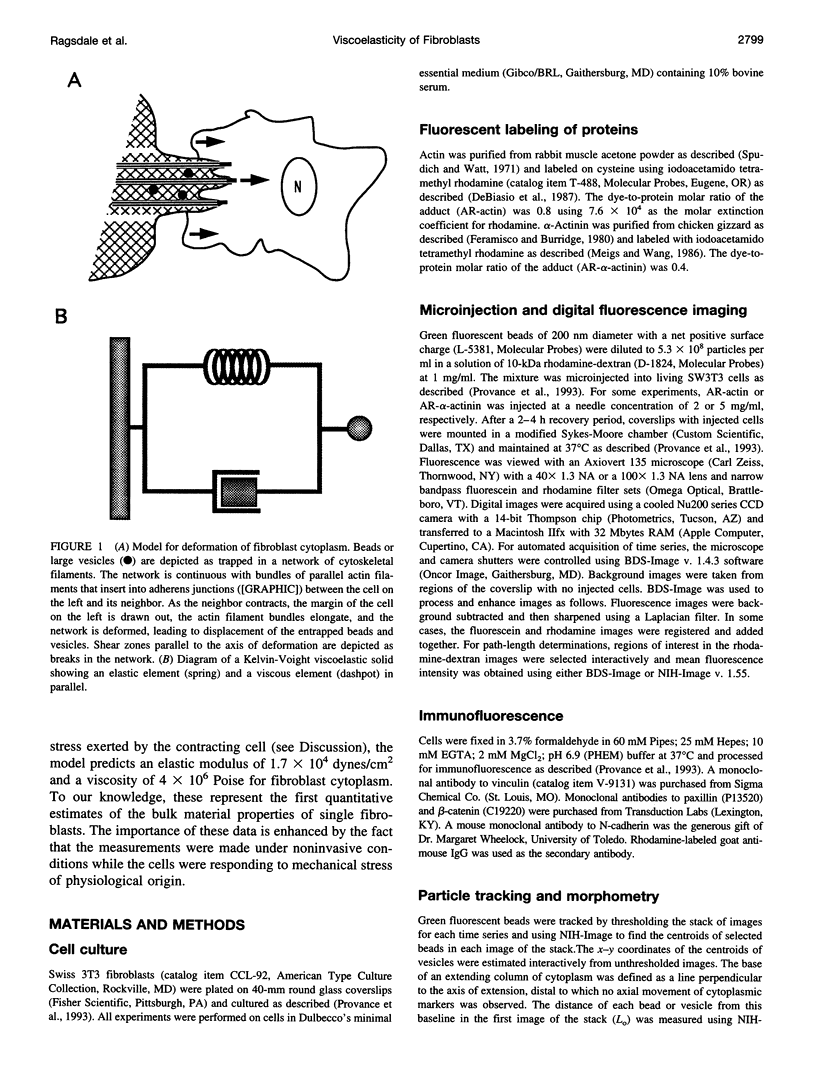

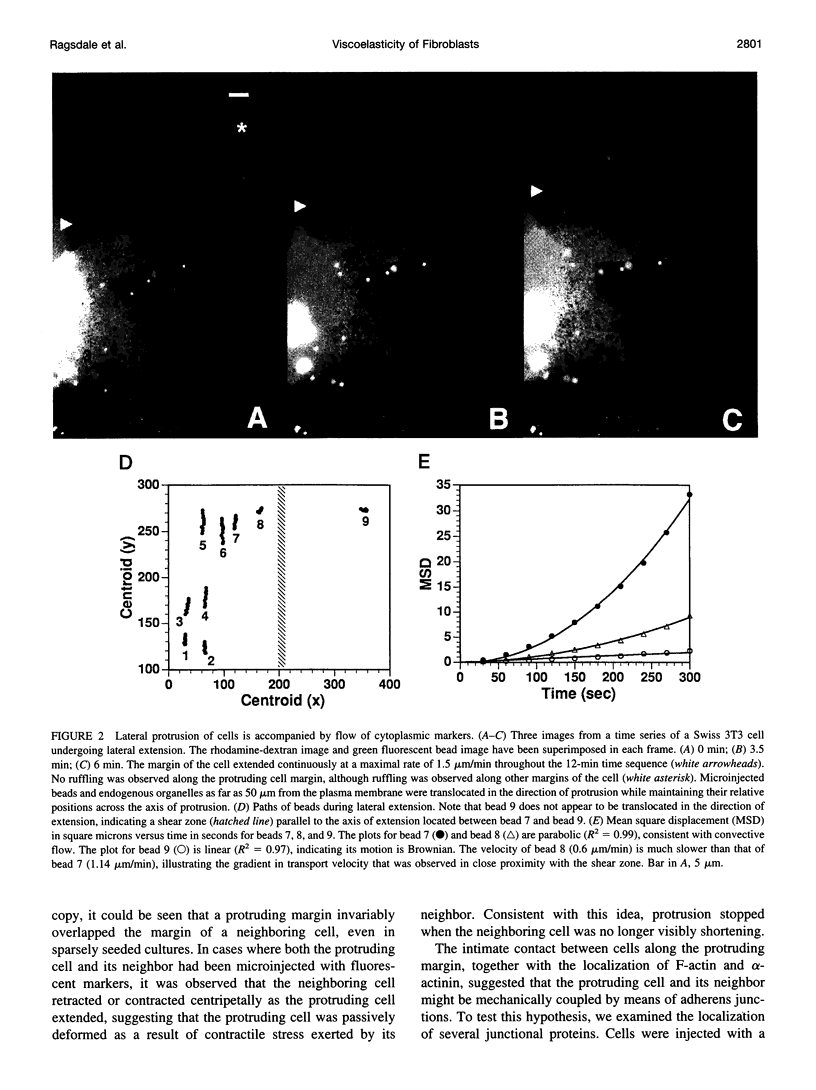
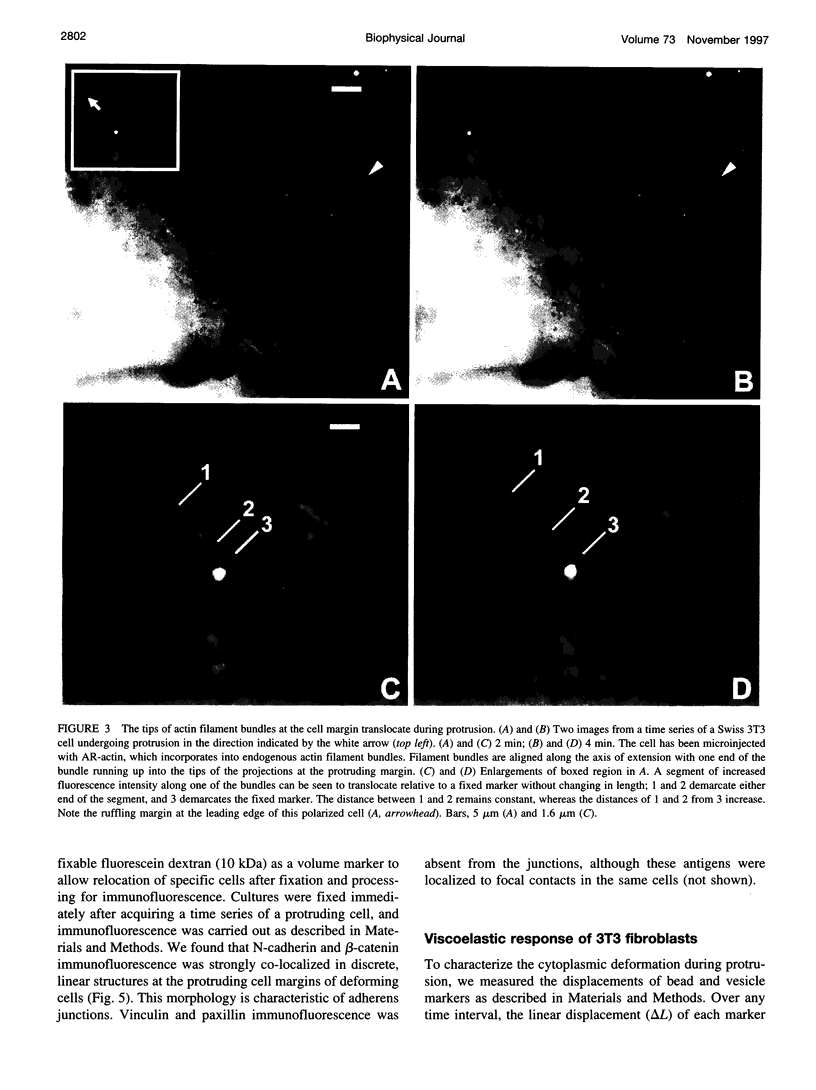
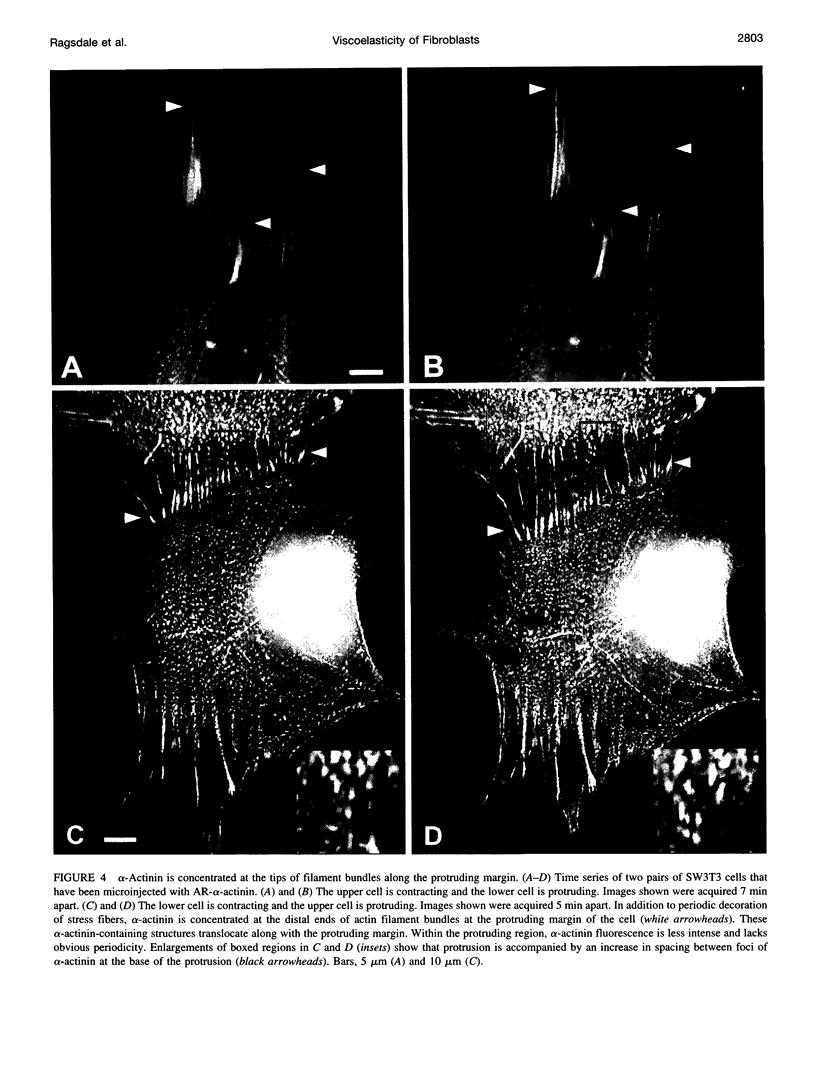


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerle M. C. Microinjected fluorescent polystyrene beads exhibit saltatory motion in tissue culture cells. J Cell Biol. 1984 Jun;98(6):2126–2132. doi: 10.1083/jcb.98.6.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizal C. L., Butler J. P., Valberg P. A. Viscoelastic and motile properties of hamster lung and peritoneal macrophages. J Leukoc Biol. 1991 Sep;50(3):240–251. doi: 10.1002/jlb.50.3.240. [DOI] [PubMed] [Google Scholar]
- Boal DH, Seifert U, Shillcock JC. Negative Poisson ratio in two-dimensional networks under tension. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993 Dec;48(6):4274–4283. doi: 10.1103/physreve.48.4274. [DOI] [PubMed] [Google Scholar]
- Chien S., Schmid-Schönbein G. W., Sung K. L., Schmalzer E. A., Skalak R. Viscoelastic properties of leukocytes. Kroc Found Ser. 1984;16:19–51. [PubMed] [Google Scholar]
- Danowski B. A., Harris A. K. Changes in fibroblast contractility, morphology, and adhesion in response to a phorbol ester tumor promoter. Exp Cell Res. 1988 Jul;177(1):47–59. doi: 10.1016/0014-4827(88)90024-9. [DOI] [PubMed] [Google Scholar]
- DeBiasio R., Bright G. R., Ernst L. A., Waggoner A. S., Taylor D. L. Five-parameter fluorescence imaging: wound healing of living Swiss 3T3 cells. J Cell Biol. 1987 Oct;105(4):1613–1622. doi: 10.1083/jcb.105.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson E. L. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Biophys Chem. 1988;17:397–430. doi: 10.1146/annurev.bb.17.060188.002145. [DOI] [PubMed] [Google Scholar]
- Evans E., Leung A., Zhelev D. Synchrony of cell spreading and contraction force as phagocytes engulf large pathogens. J Cell Biol. 1993 Sep;122(6):1295–1300. doi: 10.1083/jcb.122.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S., Elson E. L. Mechanics of fibroblast locomotion: quantitative analysis of forces and motions at the leading lamellas of fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 1):2513–2526. doi: 10.1083/jcb.111.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Burridge K. A rapid purification of alpha-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980 Feb 10;255(3):1194–1199. [PubMed] [Google Scholar]
- Feramisco J. R. Microinjection of fluorescently labeled alpha-actinin into living fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3967–3971. doi: 10.1073/pnas.76.8.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts H., De Brabander M., Nuydens R., Geuens S., Moeremans M., De Mey J., Hollenbeck P. Nanovid tracking: a new automatic method for the study of mobility in living cells based on colloidal gold and video microscopy. Biophys J. 1987 Nov;52(5):775–782. doi: 10.1016/S0006-3495(87)83271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaysman J. E., Pegrum S. M. Early contacts between fibroblasts. An ultrastructural study. Exp Cell Res. 1973 Mar 30;78(1):71–78. doi: 10.1016/0014-4827(73)90039-6. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of the cortex before and during cleavage. Ann N Y Acad Sci. 1990;582:22–30. doi: 10.1111/j.1749-6632.1990.tb21664.x. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Dike L., Hansen L., Karp S., Liley H., Maniotis A., McNamee H., Mooney D., Plopper G., Sims J. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int Rev Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Euteneuer U., Traub P., Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991 Apr;113(1):155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Hvidt S., Käs J., Lerche D., Maggs A., Sackmann E., Schliwa M., Stossel T. P. The mechanical properties of actin gels. Elastic modulus and filament motions. J Biol Chem. 1994 Dec 23;269(51):32503–32513. [PubMed] [Google Scholar]
- Knudsen K. A., Soler A. P., Johnson K. R., Wheelock M. J. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995 Jul;130(1):67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodney M. S., Wysolmerski R. B. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992 Apr;117(1):73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Leonard M., Oliver T., Ishihara A., Jacobson K. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994 Dec;127(6 Pt 2):1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Y., Young J. I., Elson E. L. Rat basophilic leukemia cells stiffen when they secrete. J Cell Biol. 1987 Dec;105(6 Pt 2):2933–2943. doi: 10.1083/jcb.105.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs J. B., Wang Y. L. Reorganization of alpha-actinin and vinculin induced by a phorbol ester in living cells. J Cell Biol. 1986 Apr;102(4):1430–1438. doi: 10.1083/jcb.102.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance D. W., Jr, McDowall A., Marko M., Luby-Phelps K. Cytoarchitecture of size-excluding compartments in living cells. J Cell Sci. 1993 Oct;106(Pt 2):565–577. doi: 10.1242/jcs.106.2.565. [DOI] [PubMed] [Google Scholar]
- Qian H., Sheetz M. P., Elson E. L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys J. 1991 Oct;60(4):910–921. doi: 10.1016/S0006-3495(91)82125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmacher M., Fritz M., Kacher C. M., Cleveland J. P., Hansma P. K. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys J. 1996 Jan;70(1):556–567. doi: 10.1016/S0006-3495(96)79602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H. Stretch-activated ion channels. Kidney Int. 1995 Oct;48(4):1134–1147. doi: 10.1038/ki.1995.397. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Mittal B., Pochapin M. B., Sanger J. W. Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin. J Cell Biol. 1986 Jun;102(6):2053–2066. doi: 10.1083/jcb.102.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Wong T. Z., Brown D. T., Allen R. D. Rheological properties of living cytoplasm: a preliminary investigation of squid axoplasm (Loligo pealei). Cell Motil. 1984;4(1):7–23. doi: 10.1002/cm.970040103. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. I., Schmid-Schönbein G. W. Kinematics of cytoplasmic deformation in neutrophils during active motion. J Biomech Eng. 1990 Aug;112(3):303–310. doi: 10.1115/1.2891188. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995 Oct;7(5):619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tsai M. A., Frank R. S., Waugh R. E. Passive mechanical behavior of human neutrophils: effect of cytochalasin B. Biophys J. 1994 Jun;66(6):2166–2172. doi: 10.1016/S0006-3495(94)81012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P. Microinjected carboxylated beads move predominantly poleward in sea urchin eggs. Cell Motil Cytoskeleton. 1987;8(4):293–301. doi: 10.1002/cm.970080402. [DOI] [PubMed] [Google Scholar]
- Wang K., McCarter R., Wright J., Beverly J., Ramirez-Mitchell R. Viscoelasticity of the sarcomere matrix of skeletal muscles. The titin-myosin composite filament is a dual-stage molecular spring. Biophys J. 1993 Apr;64(4):1161–1177. doi: 10.1016/S0006-3495(93)81482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Zahalak G. I., McConnaughey W. B., Elson E. L. Determination of cellular mechanical properties by cell poking, with an application to leukocytes. J Biomech Eng. 1990 Aug;112(3):283–294. doi: 10.1115/1.2891186. [DOI] [PubMed] [Google Scholar]
- Zaner K. S., Valberg P. A. Viscoelasticity of F-actin measured with magnetic microparticles. J Cell Biol. 1989 Nov;109(5):2233–2243. doi: 10.1083/jcb.109.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]