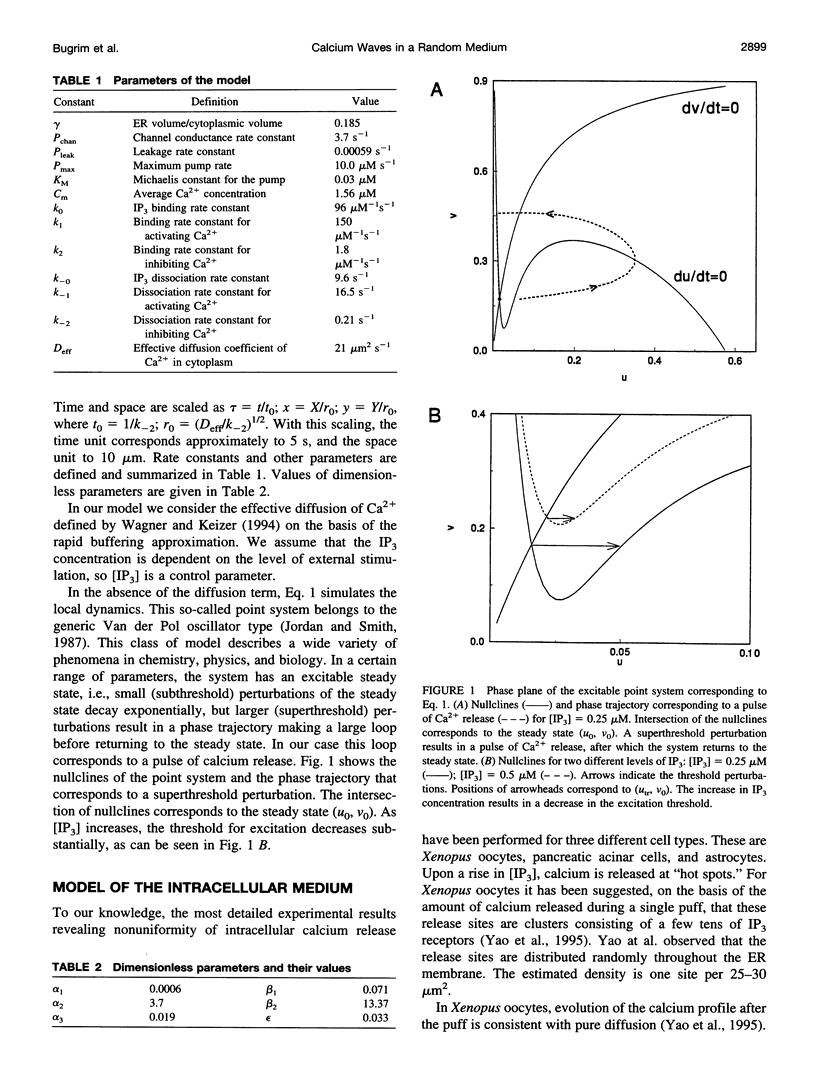
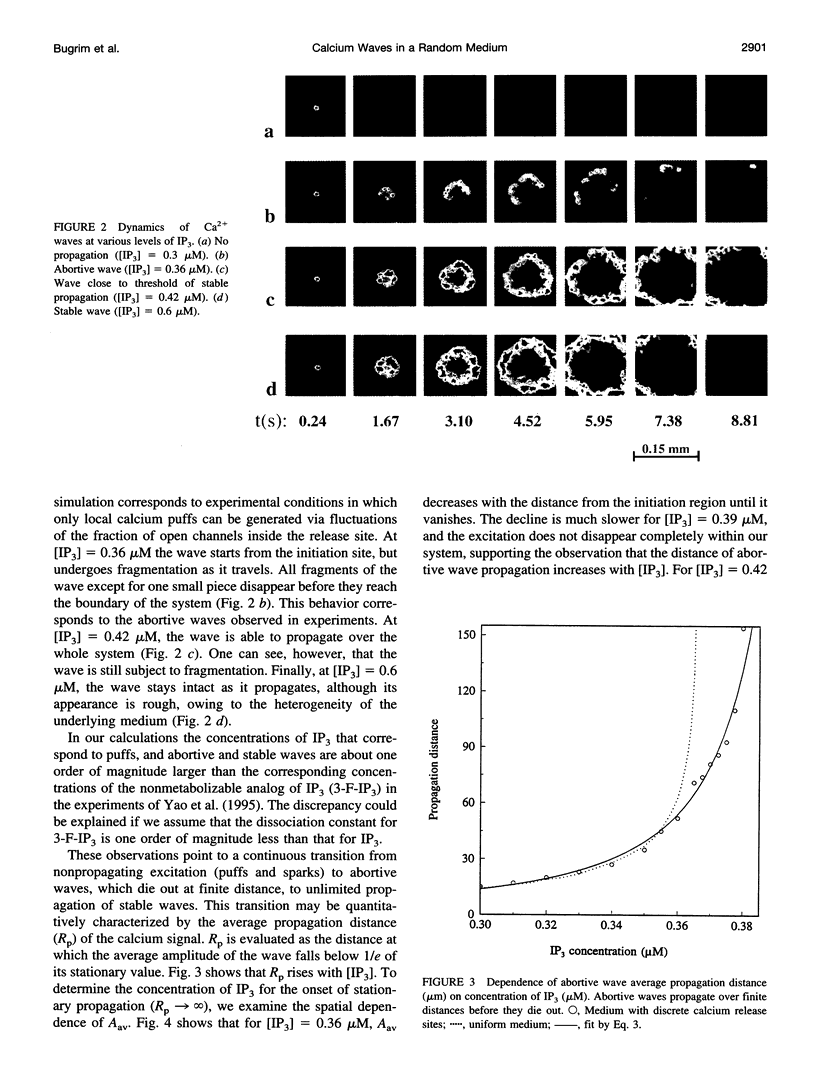
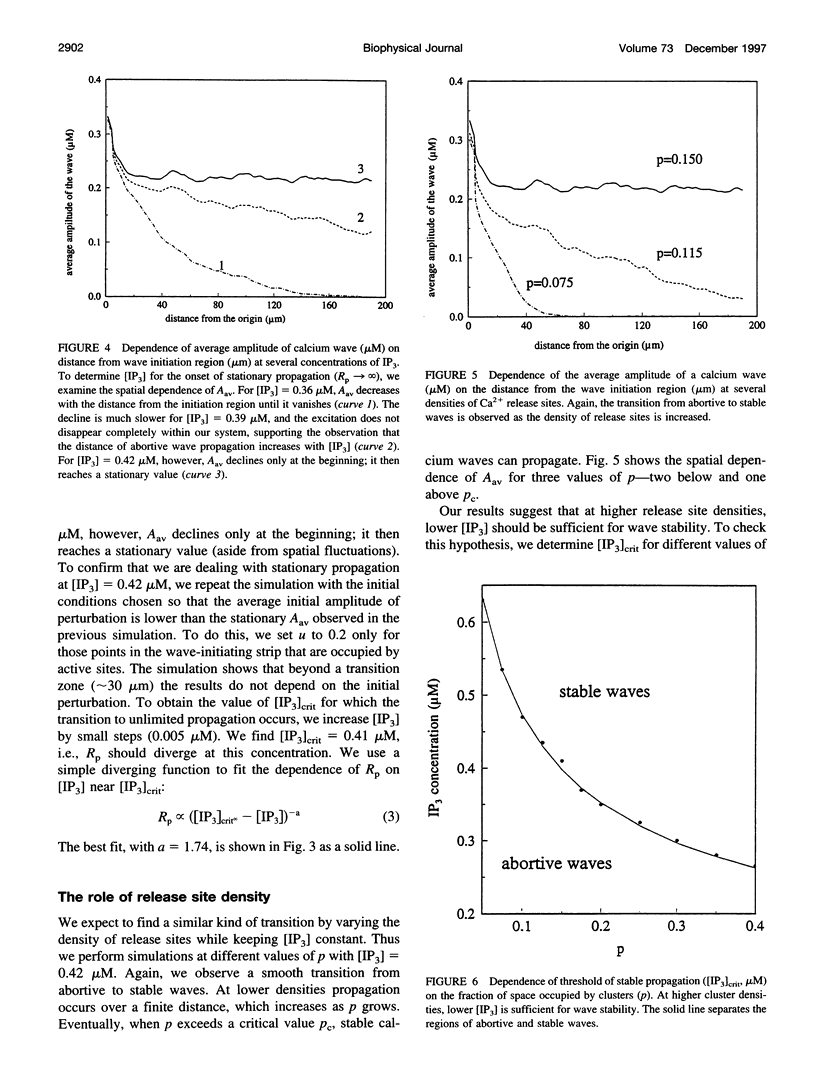
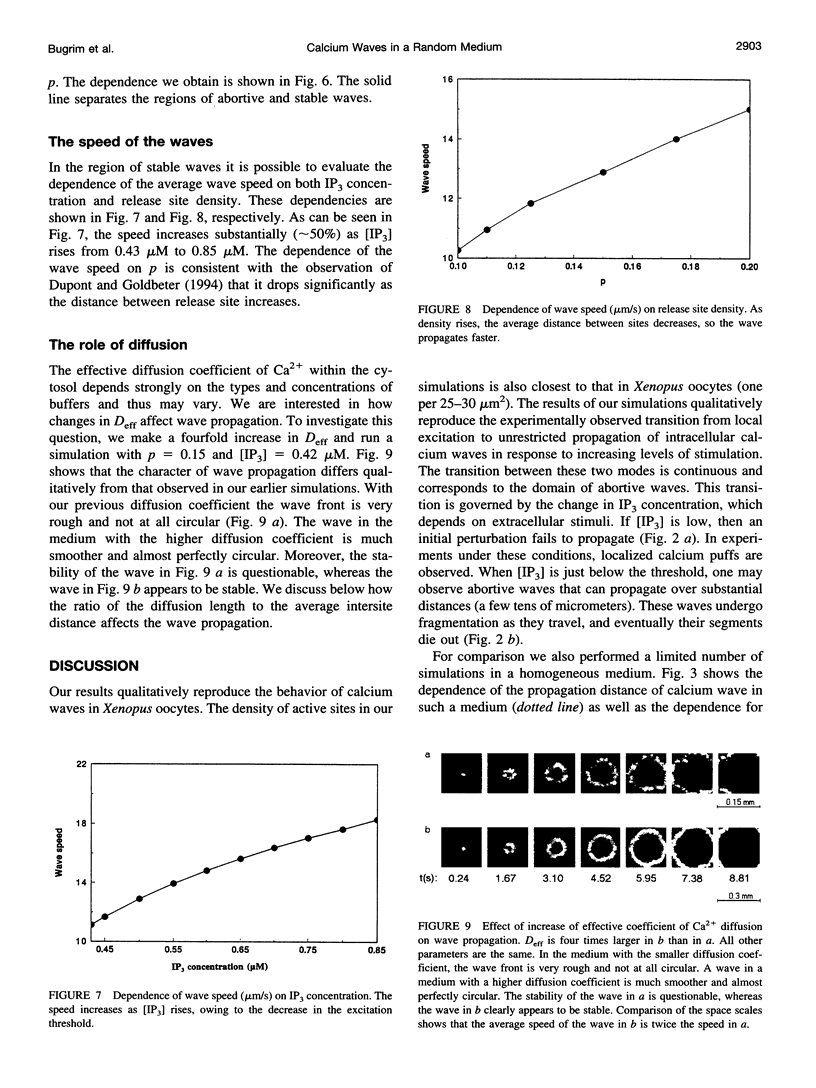
Abstract
We study the propagation of intracellular calcium waves in a model that features Ca2+ release from discrete sites in the endoplasmic reticulum membrane and random spatial distribution of these sites. The results of our simulations qualitatively reproduce the experimentally observed behavior of the waves. When the level of the channel activator inositol trisphosphate is low, the wave undergoes fragmentation and eventually vanishes at a finite distance from the region of initiation, a phenomenon we refer to as an abortive wave. With increasing activator concentration, the mean distance of propagation increases. Above a critical level of activator, the wave becomes stable. We show that the heterogeneous distribution of Ca2+ channels is the cause of this phenomenon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993 Oct;65(4):1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Elementary and global aspects of calcium signalling. J Physiol. 1997 Mar 1;499(Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer M. R., Lederer W. J., Cannell M. B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell. 1995 Jan 27;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. Properties of intracellular Ca2+ waves generated by a model based on Ca(2+)-induced Ca2+ release. Biophys J. 1994 Dec;67(6):2191–2204. doi: 10.1016/S0006-3495(94)80705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. X., Rinzel J. Equations for InsP3 receptor-mediated [Ca2+]i oscillations derived from a detailed kinetic model: a Hodgkin-Huxley like formalism. J Theor Biol. 1994 Feb 21;166(4):461–473. doi: 10.1006/jtbi.1994.1041. [DOI] [PubMed] [Google Scholar]
- Parker I., Choi J., Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996 Aug;20(2):105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- Parker I., Yao Y. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J Physiol. 1996 Mar 15;491(Pt 3):663–668. doi: 10.1113/jphysiol.1996.sp021247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991 Dec 23;246(1317):269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- Provatas N, Ala-Nissila T, Grant M, Elder KR, Piché L. Flame propagation in random media. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1995 May;51(5):4232–4236. doi: 10.1103/physreve.51.4232. [DOI] [PubMed] [Google Scholar]
- Roth B. J., Yagodin S. V., Holtzclaw L., Russell J. T. A mathematical model of agonist-induced propagation of calcium waves in astrocytes. Cell Calcium. 1995 Jan;17(1):53–64. doi: 10.1016/0143-4160(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Tang Y., Stephenson J. L., Othmer H. G. Simplification and analysis of models of calcium dynamics based on IP3-sensitive calcium channel kinetics. Biophys J. 1996 Jan;70(1):246–263. doi: 10.1016/S0006-3495(96)79567-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Moreton R., Berridge M. Multiple, coordinated Ca2+ -release events underlie the inositol trisphosphate-induced local Ca2+ spikes in mouse pancreatic acinar cells. EMBO J. 1996 Mar 1;15(5):999–1003. [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys J. 1994 Jul;67(1):447–456. doi: 10.1016/S0006-3495(94)80500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J., Bezprozvanny I., Ehrlich B. E. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductance states. J Neurosci. 1991 Oct;11(10):3239–3245. doi: 10.1523/JNEUROSCI.11-10-03239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagodin S. V., Holtzclaw L., Sheppard C. A., Russell J. T. Nonlinear propagation of agonist-induced cytoplasmic calcium waves in single astrocytes. J Neurobiol. 1994 Mar;25(3):265–280. doi: 10.1002/neu.480250307. [DOI] [PubMed] [Google Scholar]
- Yagodin S., Holtzclaw L. A., Russell J. T. Subcellular calcium oscillators and calcium influx support agonist-induced calcium waves in cultured astrocytes. Mol Cell Biochem. 1995 Aug-Sep;149-150:137–144. doi: 10.1007/BF01076572. [DOI] [PubMed] [Google Scholar]
- Yao Y., Choi J., Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol. 1995 Feb 1;482(Pt 3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]




