Abstract
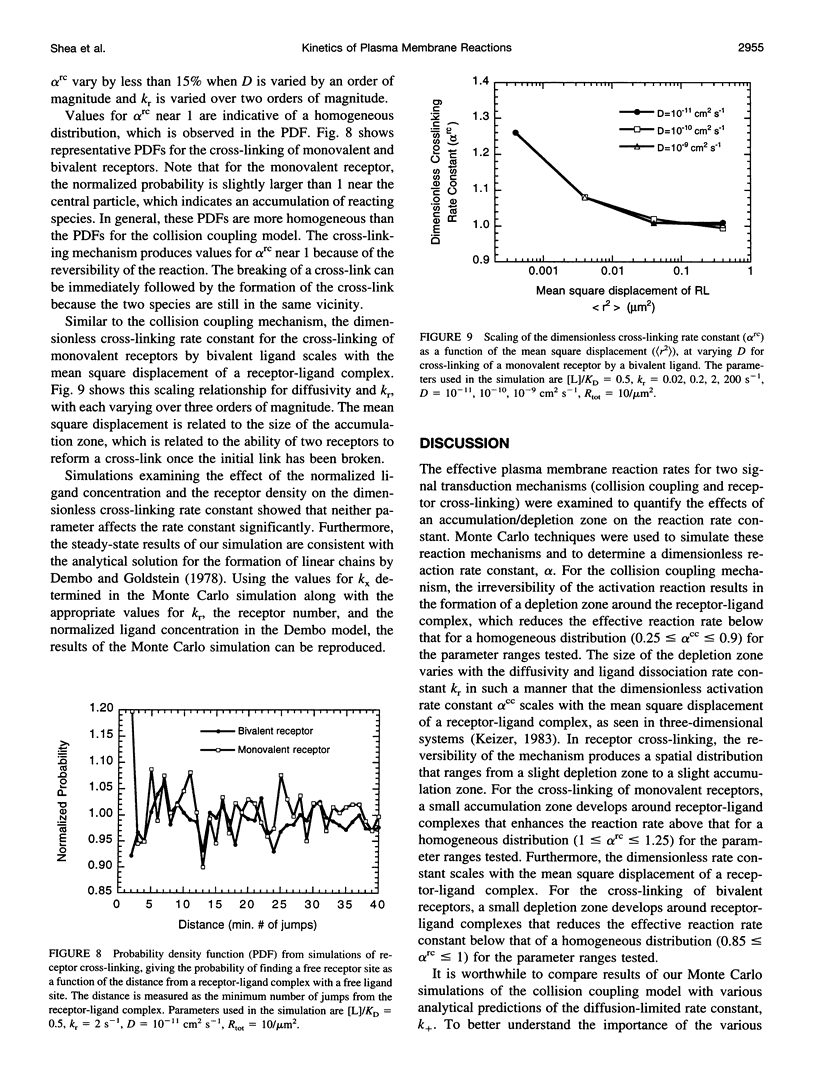
Both enzyme (e.g., G-protein) activation via a collision coupling model and the formation of cross-linked receptors by a multivalent ligand involve reactions between two molecules diffusing in the plasma membrane. The diffusion of these molecules is thought to play a critical role in these two early signal transduction events. In reduced dimensions, however, diffusion is not an effective mixing mechanism; consequently, zones in which the concentration of particular molecules (e.g., enzymes, receptors) becomes depleted or enriched may form. To examine the formation of these depletion/ accumulation zones and their effect on reaction rates and ultimately the cellular response, Monte Carlo techniques are used to simulate the reaction and diffusion of molecules in the plasma membrane. The effective reaction rate at steady state is determined in terms of the physical properties of the tissue and ligand for both enzyme activation via collision coupling and the generation of cross-linked receptors. The diffusion-limited reaction rate constant is shown to scale with the mean square displacement of a receptor-ligand complex. The rate constants determined in the simulation are compared with other theoretical predictions as well as experimental data.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas D., Volsky D. J., Levitzki A. Lateral mobility of beta-receptors involved in adenylate cyclase activation. Biochim Biophys Acta. 1980 Mar 27;597(1):64–69. doi: 10.1016/0005-2736(80)90150-9. [DOI] [PubMed] [Google Scholar]
- Bakardjieva A., Galla H. J., Helmreich E. J. Modulation of the beta-receptor adenylate cyclase interactions in cultured Chang liver cells by phospholipid enrichment. Biochemistry. 1979 Jul 10;18(14):3016–3023. doi: 10.1021/bi00581a017. [DOI] [PubMed] [Google Scholar]
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
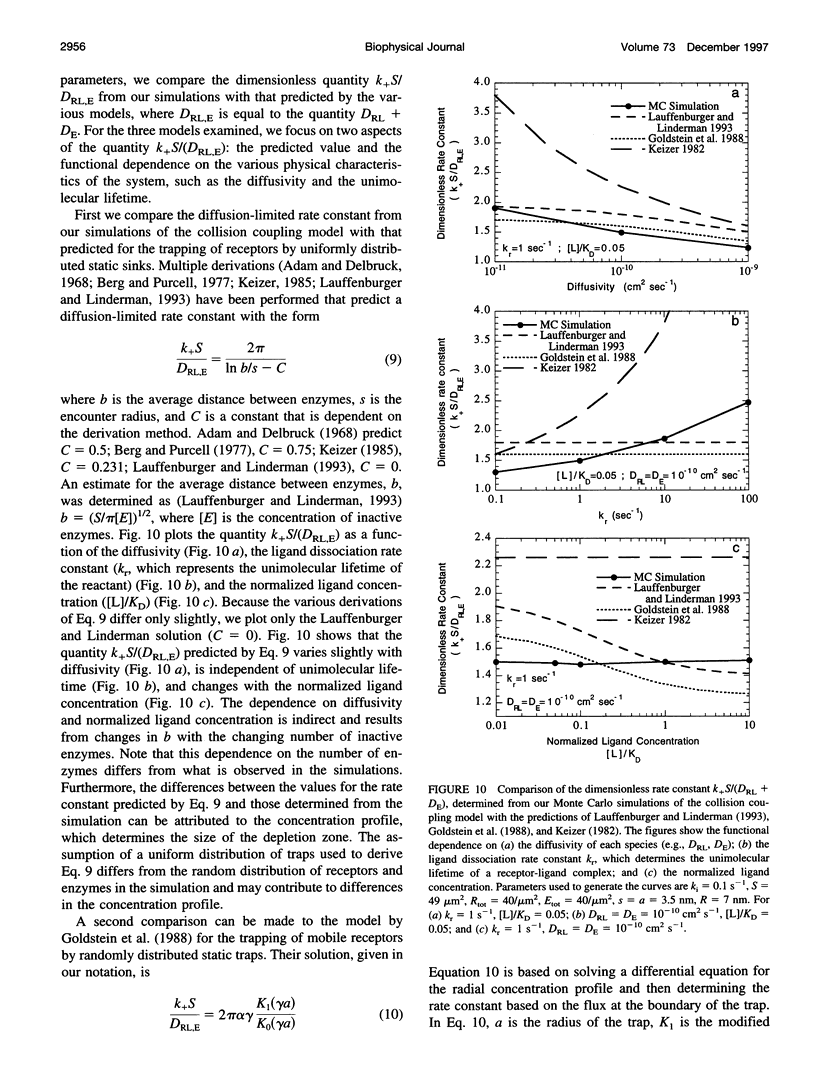
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Dembo M., Goldstein B. Theory of equilibrium binding of symmetric bivalent haptens to cell surface antibody: application to histamine release from basophils. J Immunol. 1978 Jul;121(1):345–353. [PubMed] [Google Scholar]
- Dembo M., Kagey-Sobotka A., Lichtenstein L. M., Goldstein B. Kinetic analysis of histamine release due to covalently linked IgE dimers. Mol Immunol. 1982 Mar;19(3):421–434. doi: 10.1016/0161-5890(82)90208-5. [DOI] [PubMed] [Google Scholar]
- Fichthorn K, Gulari E, Ziff R. Noise-induced bistability in a Monte Carlo surface-reaction model. Phys Rev Lett. 1989 Oct 2;63(14):1527–1530. doi: 10.1103/PhysRevLett.63.1527. [DOI] [PubMed] [Google Scholar]
- Goldstein B., Wofsy C., Bell G. Interactions of low density lipoprotein receptors with coated pits on human fibroblasts: estimate of the forward rate constant and comparison with the diffusion limit. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5695–5698. doi: 10.1073/pnas.78.9.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B., Wofsy C., Echavarría-Heras H. Effect of membrane flow on the capture of receptors by coated pits. Theoretical results. Biophys J. 1988 Mar;53(3):405–414. doi: 10.1016/S0006-3495(88)83117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe W. C., Conn P. M. Membrane fluidity regulates development of gonadotrope desensitization to GnRH. Mol Cell Endocrinol. 1987 Sep;53(1-2):131–140. doi: 10.1016/0303-7207(87)90199-7. [DOI] [PubMed] [Google Scholar]
- Hanski E., Rimon G., Levitzki A. Adenylate cyclase activation by the beta-adrenergic receptors as a diffusion-controlled process. Biochemistry. 1979 Mar 6;18(5):846–853. doi: 10.1021/bi00572a017. [DOI] [PubMed] [Google Scholar]
- Jans D. A. The mobile receptor hypothesis revisited: a mechanistic role for hormone receptor lateral mobility in signal transduction. Biochim Biophys Acta. 1992 Dec 11;1113(3-4):271–276. doi: 10.1016/0304-4157(92)90001-q. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. Stochastic simulation of activation in the G-protein cascade of phototransduction. Biophys J. 1994 Oct;67(4):1439–1454. doi: 10.1016/S0006-3495(94)80617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahama P. A., Linderman J. J. A Monte Carlo study of the dynamics of G-protein activation. Biophys J. 1994 Sep;67(3):1345–1357. doi: 10.1016/S0006-3495(94)80606-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahama P. A., Linderman J. J. Monte Carlo simulations of membrane signal transduction events: effect of receptor blockers on G-protein activation. Ann Biomed Eng. 1995 May-Jun;23(3):299–307. doi: 10.1007/BF02584430. [DOI] [PubMed] [Google Scholar]
- Malveaux F. J., Conroy M. C., Adkinson N. F., Jr, Lichtenstein L. M. IgE receptors on human basophils. Relationship to serum IgE concentration. J Clin Invest. 1978 Jul;62(1):176–181. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Holowka D., Webb W. W., Baird B. Cross-linking of receptor-bound IgE to aggregates larger than dimers leads to rapid immobilization. J Cell Biol. 1986 Feb;102(2):541–550. doi: 10.1083/jcb.102.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995 Jan 27;80(2):249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Posner R. G., Subramanian K., Goldstein B., Thomas J., Feder T., Holowka D., Baird B. Simultaneous cross-linking by two nontriggering bivalent ligands causes synergistic signaling of IgE Fc epsilon RI complexes. J Immunol. 1995 Oct 1;155(7):3601–3609. [PubMed] [Google Scholar]
- Post S. R., Hilal-Dandan R., Urasawa K., Brunton L. L., Insel P. A. Quantification of signalling components and amplification in the beta-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J. 1995 Oct 1;311(Pt 1):75–80. doi: 10.1042/bj3110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J., Owicki J. C. Concentration effects on reactions in membranes: rhodopsin and transducin. Biochim Biophys Acta. 1989 Feb 13;979(1):27–34. doi: 10.1016/0005-2736(89)90519-1. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Shea L., Linderman J. J. Mechanistic model of G-protein signal transduction. Determinants of efficacy and effect of precoupled receptors. Biochem Pharmacol. 1997 Feb 21;53(4):519–530. doi: 10.1016/s0006-2952(96)00768-x. [DOI] [PubMed] [Google Scholar]
- Sklar L. A. Ligand-receptor dynamics and signal amplification in the neutrophil. Adv Immunol. 1986;39:95–143. doi: 10.1016/s0065-2776(08)60349-1. [DOI] [PubMed] [Google Scholar]
- Stickle D., Barber R. Comparisons of the combined contributions of agonist binding frequency and intrinsic efficiency to receptor-mediated activation of adenylate cyclase. Mol Pharmacol. 1991 Aug;40(2):276–288. [PubMed] [Google Scholar]
- Stickle D., Barber R. Evidence for the role of epinephrine binding frequency in activation of adenylate cyclase. Mol Pharmacol. 1989 Sep;36(3):437–445. [PubMed] [Google Scholar]
- Subramanian K., Holowka D., Baird B., Goldstein B. The Fc segment of IgE influences the kinetics of dissociation of a symmetrical bivalent ligand from cyclic dimeric complexes. Biochemistry. 1996 Apr 30;35(17):5518–5527. doi: 10.1021/bi9523522. [DOI] [PubMed] [Google Scholar]
- Taylor C. W. The role of G proteins in transmembrane signalling. Biochem J. 1990 Nov 15;272(1):1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978 Sep 5;17(18):3795–3795. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- Wickham T. J., Granados R. R., Wood H. A., Hammer D. A., Shuler M. L. General analysis of receptor-mediated viral attachment to cell surfaces. Biophys J. 1990 Dec;58(6):1501–1516. doi: 10.1016/S0006-3495(90)82495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy C., Goldstein B., Dembo M. Theory of equilibrium binding of asymmetric bivalent haptens to cell surface antibody: application to histamine release from basophils. J Immunol. 1978 Aug;121(2):593–601. [PubMed] [Google Scholar]


