Abstract
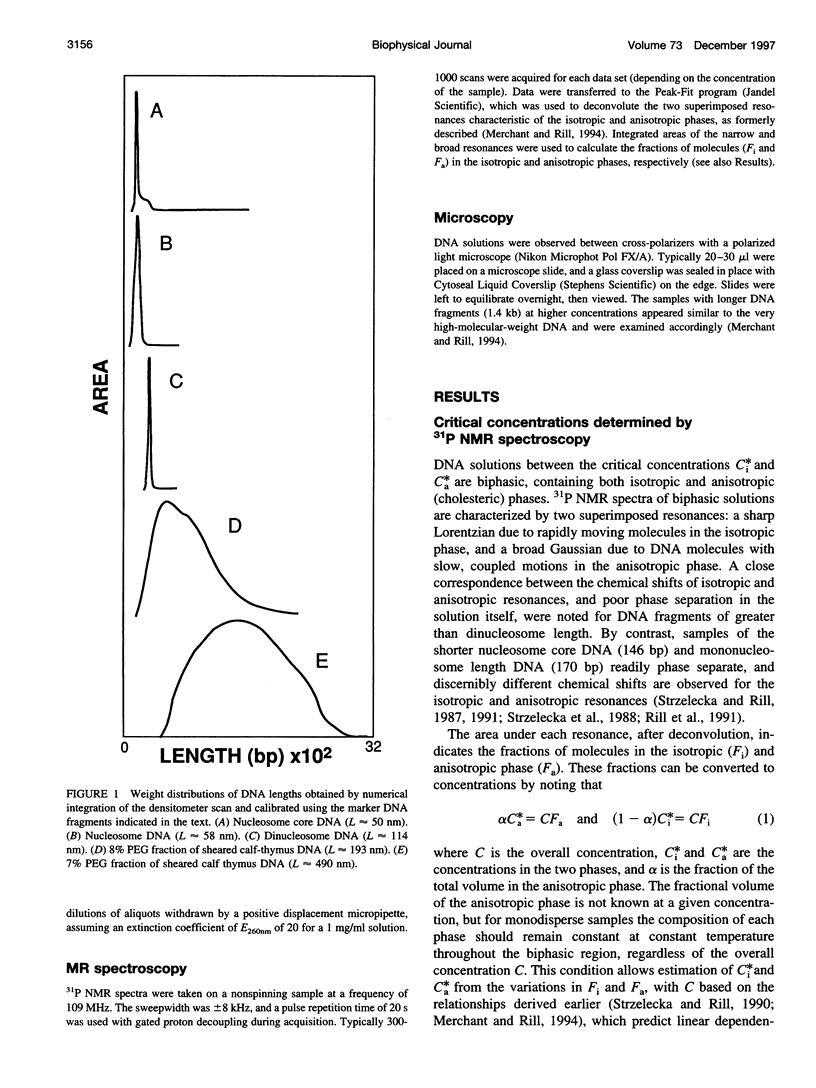
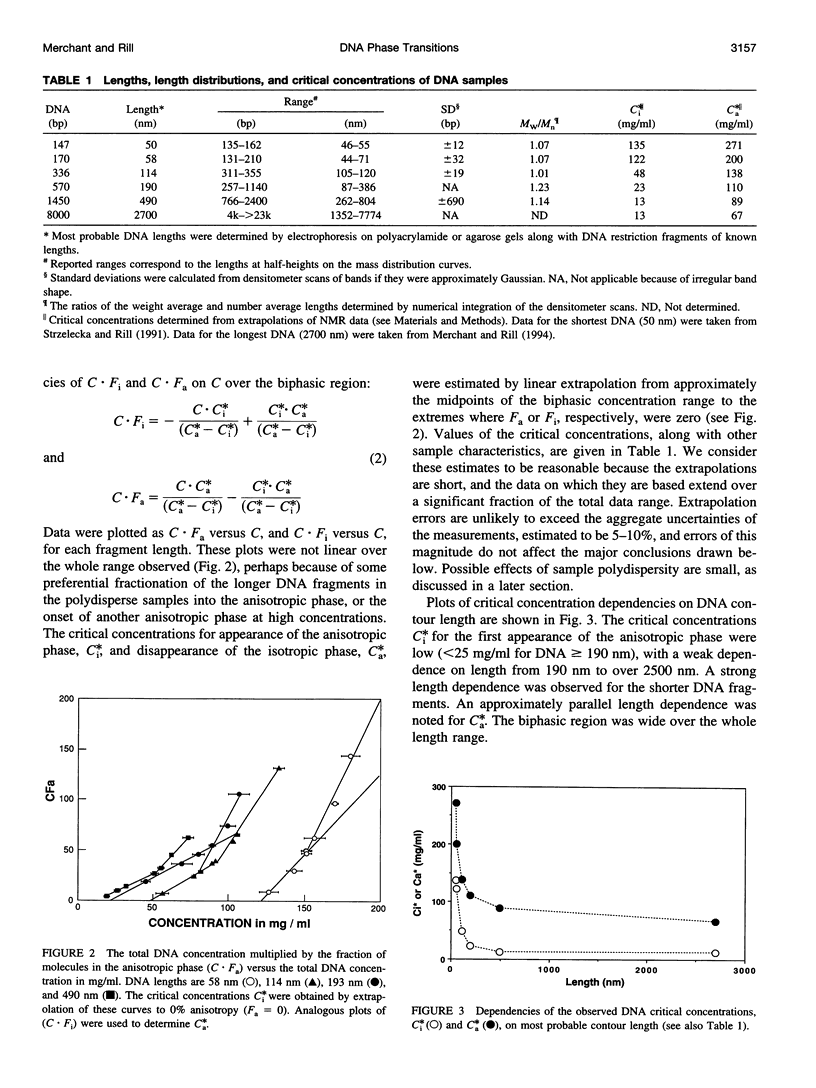
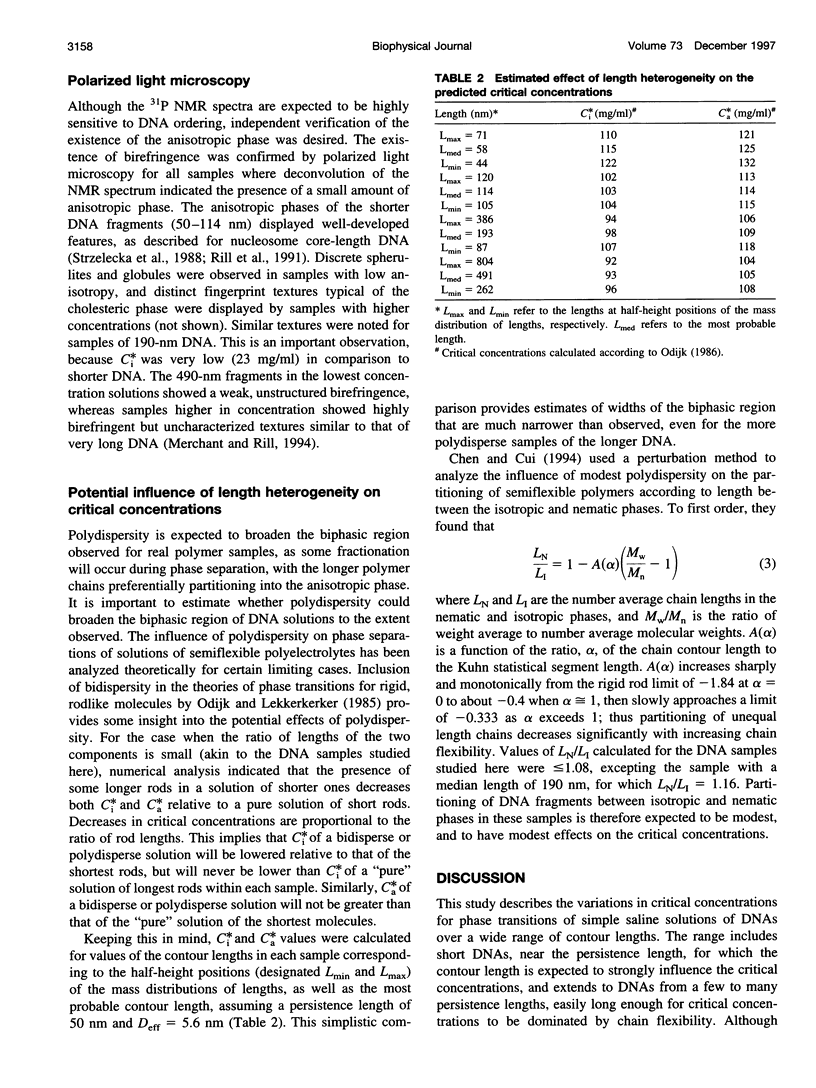
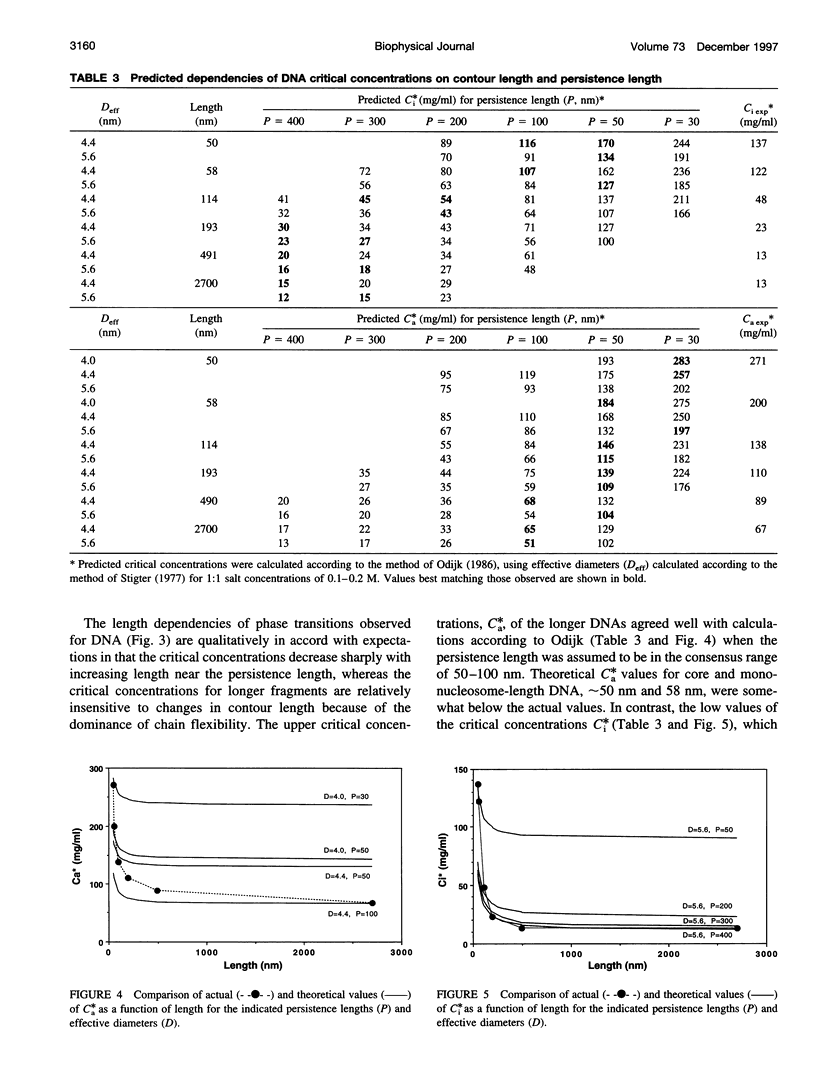
Critical concentrations for the isotropic to cholesteric phase transitions of double-stranded DNA fragments in simple buffered saline (0.1 M NaCl) solutions were determined as a function of DNA contour length ranging from approximately 50 nm to 2700 nm, by solid-state 31P NMR spectroscopy and polarized light microscopy. As expected for semirigid chains, the critical concentrations decrease sharply with increasing DNA length near the persistence length in the range from 50 to 110 nm, and approach a plateau when the contour length exceeds 190 nm. The biphasic region is substantially wider than observed for xanthan, another semirigid polyelectrolyte approximately twice as stiff as DNA, primarily because of low critical concentrations for first appearance of the anisotropic phase, C(i)*, in DNA samples > or =110 nm (320 base pairs) long. The limiting C(i)* for DNA > or =490 nm long is exceptionally low (only 13 mg/ml) and is substantially lower than the C(i)* of approximately 40 mg/ml reported for the stiffer xanthan polyelectrolyte. The much higher values of the critical concentrations, C(a)*, for the disappearance of the isotropic DNA phase (> or =67 mg/ml) are modestly higher than those observed for xanthan and are predicted reasonably well by a theory that has been applied to other semirigid polymers, if a DNA persistence length in the consensus range of 50-100 nm is assumed. By contrast, the broad biphasic region and low C(i)* values of DNA fragments > or =190 nm long could only be reconciled with theory by assuming persistence lengths of 200-400 nm. The latter discrepancies are presumed to reflect some combination of deficiencies in current theory as applied to chiral, strong polyelectrolytes such as DNA, and sequence-dependent variations in DNA properties such as flexibility, curvature, or interaction potential. The propensity of DNA to spontaneously self-order at low concentrations well in the physiological range may have biological significance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomfield V. A. DNA condensation. Curr Opin Struct Biol. 1996 Jun;6(3):334–341. doi: 10.1016/s0959-440x(96)80052-2. [DOI] [PubMed] [Google Scholar]
- Booy F. P., Newcomb W. W., Trus B. L., Brown J. C., Baker T. S., Steven A. C. Liquid-crystalline, phage-like packing of encapsidated DNA in herpes simplex virus. Cell. 1991 Mar 8;64(5):1007–1015. doi: 10.1016/0092-8674(91)90324-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes R., Kearns D. R. Magnetic ordering of DNA liquid crystals. Biochemistry. 1986 Oct 7;25(20):5890–5895. doi: 10.1021/bi00368a008. [DOI] [PubMed] [Google Scholar]
- Brian A. A., Frisch H. L., Lerman L. S. Thermodynamics and equilibrium sedimentation analysis of the close approach of DNA molecules and a molecular ordering transition. Biopolymers. 1981 Jun;20(6):1305–1328. doi: 10.1002/bip.1981.360200615. [DOI] [PubMed] [Google Scholar]
- Brukner I., Sánchez R., Suck D., Pongor S. Sequence-dependent bending propensity of DNA as revealed by DNase I: parameters for trinucleotides. EMBO J. 1995 Apr 18;14(8):1812–1818. doi: 10.1002/j.1460-2075.1995.tb07169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M., Drak J., Kahn J. D., Levene S. D. DNA bending, flexibility, and helical repeat by cyclization kinetics. Methods Enzymol. 1992;212:3–29. doi: 10.1016/0076-6879(92)12003-9. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Haran T. E., Nadeau J. G. Intrinsically bent DNA. J Biol Chem. 1990 May 5;265(13):7093–7096. [PubMed] [Google Scholar]
- Fried M. G., Bloomfield V. A. DNA gelation in concentrated solutions. Biopolymers. 1984 Nov;23(11 Pt 1):2141–2155. doi: 10.1002/bip.360231104. [DOI] [PubMed] [Google Scholar]
- Fujimoto B. S., Schurr J. M. Dependence of the torsional rigidity of DNA on base composition. Nature. 1990 Mar 8;344(6262):175–177. doi: 10.1038/344175a0. [DOI] [PubMed] [Google Scholar]
- Fulmer A. W., Benbasat J. A., Bloomfield V. A. Ionic strength effects on macroion diffusion and excess light-scattering intensities of short DNA rods. Biopolymers. 1981 Jun;20(6):1147–1159. doi: 10.1002/bip.1981.360200606. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Flexibility of DNA. Annu Rev Biophys Biophys Chem. 1988;17:265–286. doi: 10.1146/annurev.bb.17.060188.001405. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence-directed curvature of DNA. Annu Rev Biochem. 1990;59:755–781. doi: 10.1146/annurev.bi.59.070190.003543. [DOI] [PubMed] [Google Scholar]
- Harvey S. C., Dlakic M., Griffith J., Harrington R., Park K., Sprous D., Zacharias W. What is the basis of sequence-directed curvature in DNAs containing A tracts? J Biomol Struct Dyn. 1995 Oct;13(2):301–307. doi: 10.1080/07391102.1995.10508841. [DOI] [PubMed] [Google Scholar]
- Heath P. J., Clendenning J. B., Fujimoto B. S., Schurr J. M. Effect of bending strain on the torsion elastic constant of DNA. J Mol Biol. 1996 Aug 2;260(5):718–730. doi: 10.1006/jmbi.1996.0432. [DOI] [PubMed] [Google Scholar]
- Hustedt E. J., Spaltenstein A., Kirchner J. J., Hopkins P. B., Robinson B. H. Motions of short DNA duplexes: an analysis of DNA dynamics using an EPR-active probe. Biochemistry. 1993 Feb 23;32(7):1774–1787. doi: 10.1021/bi00058a011. [DOI] [PubMed] [Google Scholar]
- Härd T., Kearns D. R. Association of short DNA fragments: steady state fluorescence polarization study. Biopolymers. 1986 Aug;25(8):1519–1529. doi: 10.1002/bip.360250810. [DOI] [PubMed] [Google Scholar]
- Kahn J. D., Crothers D. M. Protein-induced bending and DNA cyclization. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6343–6347. doi: 10.1073/pnas.89.14.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E., Arnold-Schulz-Gahmen B. Chromatins of low-protein content: special features of their compaction and condensation. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):361–370. doi: 10.1111/j.1574-6968.1992.tb14064.x. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Martinson H. G. Nucleosomes will not form on double-stranded RNa or over poly(dA).poly(dT) tracts in recombinant DNA. Nucleic Acids Res. 1981 Dec 21;9(24):6869–6888. doi: 10.1093/nar/9.24.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUZZATI V., NICOLAIEFF A., MASSON F. [Structure of desoxyribonucleic acid in solution. Study by the diffusion of x-rays at small angles]. J Mol Biol. 1961 Apr;3:185–201. doi: 10.1016/s0022-2836(61)80045-4. [DOI] [PubMed] [Google Scholar]
- Lepault J., Dubochet J., Baschong W., Kellenberger E. Organization of double-stranded DNA in bacteriophages: a study by cryo-electron microscopy of vitrified samples. EMBO J. 1987 May;6(5):1507–1512. doi: 10.1002/j.1460-2075.1987.tb02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T. Fractionation of DNA fragments by polyethylene glycol induced precipitation. Methods Enzymol. 1980;65(1):347–353. doi: 10.1016/s0076-6879(80)65044-7. [DOI] [PubMed] [Google Scholar]
- Liu-Johnson H. N., Gartenberg M. R., Crothers D. M. The DNA binding domain and bending angle of E. coli CAP protein. Cell. 1986 Dec 26;47(6):995–1005. doi: 10.1016/0092-8674(86)90814-7. [DOI] [PubMed] [Google Scholar]
- Livolant F. Cholesteric organization of DNA in the stallion sperm head. Tissue Cell. 1984;16(4):535–555. doi: 10.1016/0040-8166(84)90029-6. [DOI] [PubMed] [Google Scholar]
- Livolant F. Cholesteric organization of DNA in vivo and in vitro. Eur J Cell Biol. 1984 Mar;33(2):300–311. [PubMed] [Google Scholar]
- Lyubchenko Y. L., Shlyakhtenko L. S., Appella E., Harrington R. E. CA runs increase DNA flexibility in the complex of lambda Cro protein with the OR3 site. Biochemistry. 1993 Apr 20;32(15):4121–4127. doi: 10.1021/bi00066a038. [DOI] [PubMed] [Google Scholar]
- Mandelkern M., Dattagupta N., Crothers D. M. Conversion of B DNA between solution and fiber conformations. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4294–4298. doi: 10.1073/pnas.78.7.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Venable J. H., Jr, Lerman L. S. The structure of psi DNA. J Mol Biol. 1974 Mar 25;84(1):37–64. doi: 10.1016/0022-2836(74)90211-3. [DOI] [PubMed] [Google Scholar]
- Olson W. K., Babcock M. S., Gorin A., Liu G., Marky N. L., Martino J. A., Pedersen S. C., Srinivasan A. R., Tobias I., Westcott T. P. Flexing and folding double helical DNA. Biophys Chem. 1995 Jun-Jul;55(1-2):7–29. doi: 10.1016/0301-4622(94)00139-b. [DOI] [PubMed] [Google Scholar]
- Olson W. K. Simulating DNA at low resolution. Curr Opin Struct Biol. 1996 Apr;6(2):242–256. doi: 10.1016/s0959-440x(96)80082-0. [DOI] [PubMed] [Google Scholar]
- Podgornik R., Strey H. H., Rau D. C., Parsegian V. A. Watching molecules crowd: DNA double helices under osmotic stress. Biophys Chem. 1995 Dec;57(1):111–121. doi: 10.1016/0301-4622(95)00058-6. [DOI] [PubMed] [Google Scholar]
- Reich Z., Levin-Zaidman S., Gutman S. B., Arad T., Minsky A. Supercoiling-regulated liquid-crystalline packaging of topologically-constrained, nucleosome-free DNA molecules. Biochemistry. 1994 Nov 29;33(47):14177–14184. doi: 10.1021/bi00251a029. [DOI] [PubMed] [Google Scholar]
- Rhodes D. Nucleosome cores reconstituted from poly (dA-dT) and the octamer of histones. Nucleic Acids Res. 1979;6(5):1805–1816. doi: 10.1093/nar/6.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rill R. L., Hilliard P. R., Jr, Levy G. C. Spontaneous ordering of DNA. Effects of intermolecular interactions on DNA motional dynamics monitored by 13C and 31P nuclear magnetic resonance spectroscopy. J Biol Chem. 1983 Jan 10;258(1):250–256. [PubMed] [Google Scholar]
- Rill R. L. Liquid crystalline phases in concentrated aqueous solutions of Na+ DNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):342–346. doi: 10.1073/pnas.83.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rill R. L., Livolant F., Aldrich H. C., Davidson M. W. Electron microscopy of liquid crystalline DNA: direct evidence for cholesteric-like organization of DNA in dinoflagellate chromosomes. Chromosoma. 1989 Oct;98(4):280–286. doi: 10.1007/BF00327314. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Drobny G. P. Site-specific dynamics in DNA: theory. Annu Rev Biophys Biomol Struct. 1995;24:523–549. doi: 10.1146/annurev.bb.24.060195.002515. [DOI] [PubMed] [Google Scholar]
- Sarai A., Mazur J., Nussinov R., Jernigan R. L. Sequence dependence of DNA conformational flexibility. Biochemistry. 1989 Sep 19;28(19):7842–7849. doi: 10.1021/bi00445a046. [DOI] [PubMed] [Google Scholar]
- Satchwell S. C., Drew H. R., Travers A. A. Sequence periodicities in chicken nucleosome core DNA. J Mol Biol. 1986 Oct 20;191(4):659–675. doi: 10.1016/0022-2836(86)90452-3. [DOI] [PubMed] [Google Scholar]
- Schellman J. A., Harvey S. C. Static contributions to the persistence length of DNA and dynamic contributions to DNA curvature. Biophys Chem. 1995 Jun-Jul;55(1-2):95–114. doi: 10.1016/0301-4622(94)00144-9. [DOI] [PubMed] [Google Scholar]
- Shrader T. E., Crothers D. M. Artificial nucleosome positioning sequences. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7418–7422. doi: 10.1073/pnas.86.19.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Künzler P. Cromatin and core particles formed from the inner histones and synthetic polydeoxyribonucleotides of defined sequence. Nucleic Acids Res. 1979 Apr;6(4):1387–1415. doi: 10.1093/nar/6.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigter D. Interactions of highly charged colloidal cylinders with applications to double-stranded. Biopolymers. 1977 Jul;16(7):1435–1448. doi: 10.1002/bip.1977.360160705. [DOI] [PubMed] [Google Scholar]
- Strzelecka T. E., Davidson M. W., Rill R. L. Multiple liquid crystal phases of DNA at high concentrations. Nature. 1988 Feb 4;331(6155):457–460. doi: 10.1038/331457a0. [DOI] [PubMed] [Google Scholar]
- Strzelecka T. E., Rill R. L. Phase transitions of concentrated DNA solutions in low concentrations of 1:1 supporting electrolyte. Biopolymers. 1990;30(1-2):57–71. doi: 10.1002/bip.360300108. [DOI] [PubMed] [Google Scholar]
- Trifonov E. N. Curved DNA. CRC Crit Rev Biochem. 1985;19(2):89–106. doi: 10.3109/10409238509082540. [DOI] [PubMed] [Google Scholar]
- Trifonov E. N., Sussman J. L. The pitch of chromatin DNA is reflected in its nucleotide sequence. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3816–3820. doi: 10.1073/pnas.77.7.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]


