Abstract
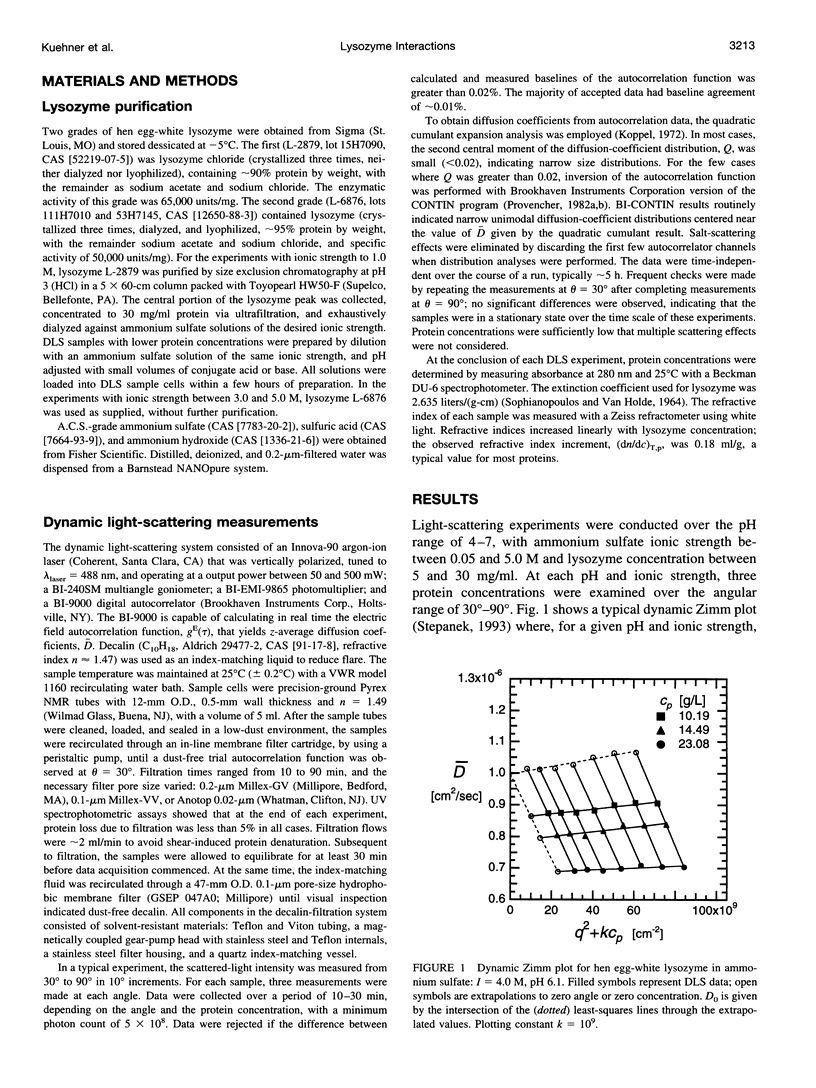
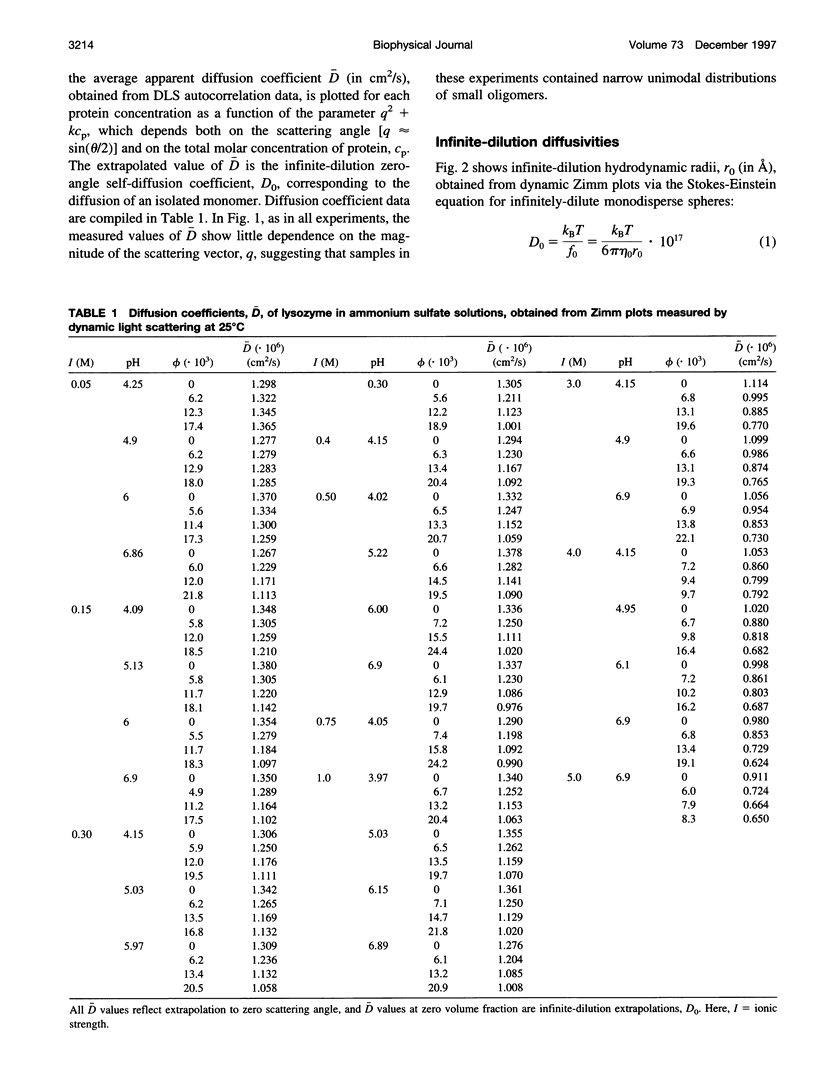
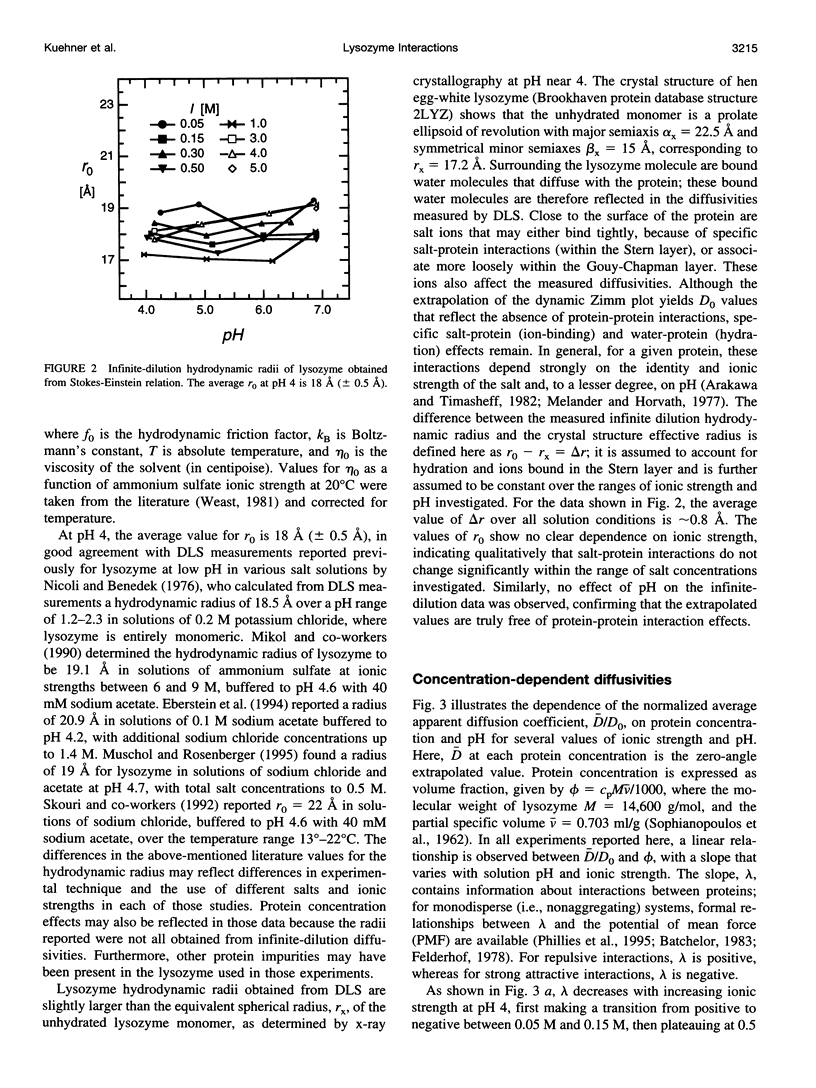
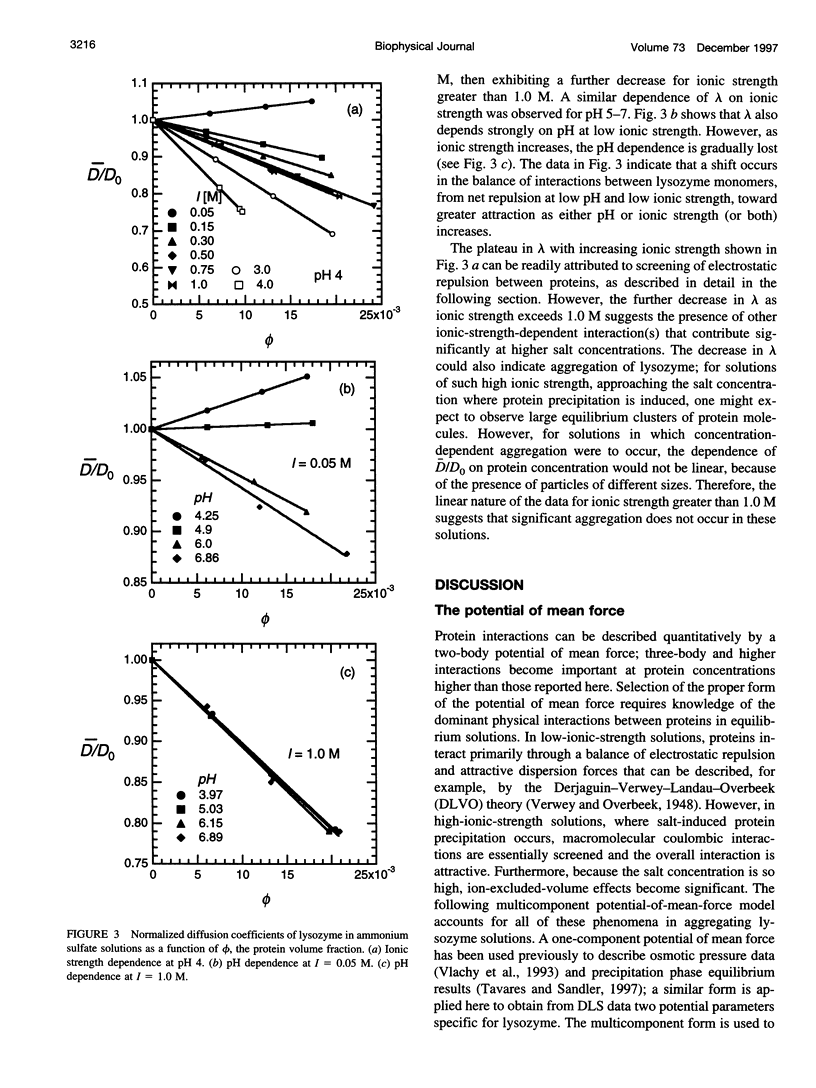
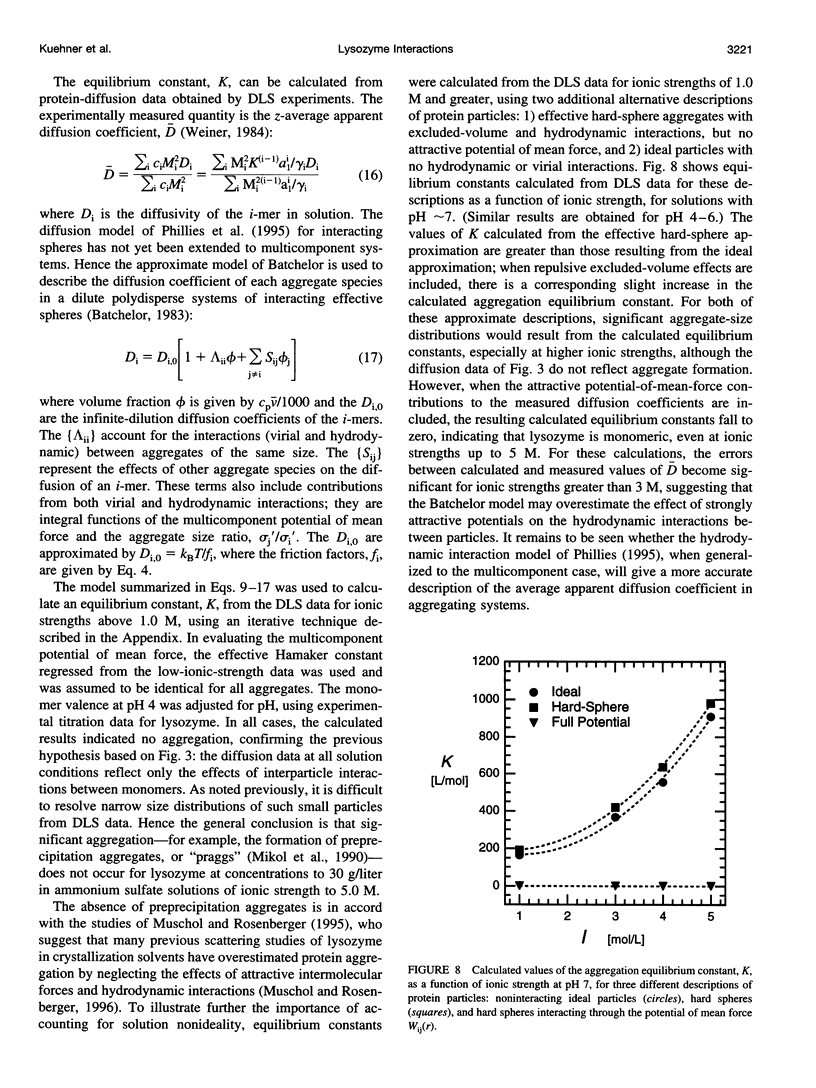
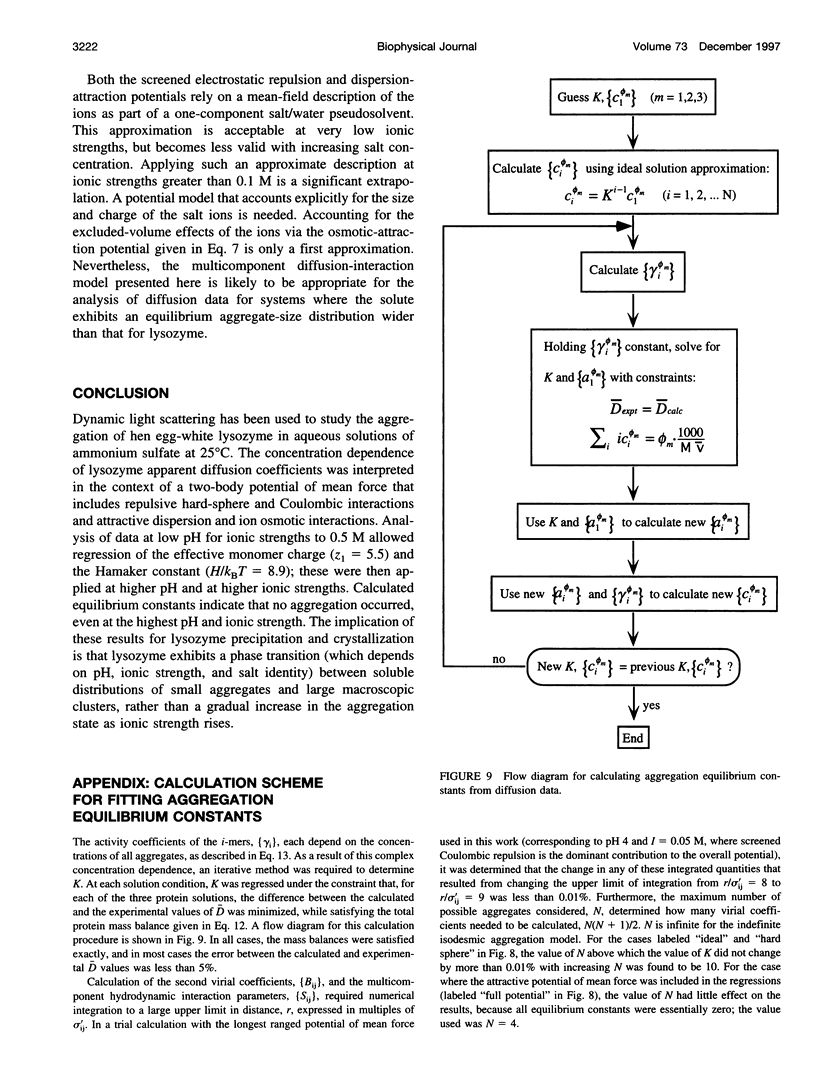
The diffusion of hen egg-white lysozyme has been studied by dynamic light scattering in aqueous solutions of ammonium sulfate as a function of protein concentration to 30 g/liter. Experiments were conducted under the following conditions: pH 4-7 and ionic strength 0.05-5.0 M. Diffusivity data for ionic strengths up to 0.5 M were interpreted in the context of a two-body interaction model for monomers. From this analysis, two potential-of-mean-force parameters, the effective monomer charge, and the Hamaker constant were obtained. At higher ionic strength, the data were analyzed using a model that describes the diffusion coefficient of a polydisperse system of interacting protein aggregates in terms of an isodesmic, indefinite aggregation equilibrium constant. Data analysis incorporated multicomponent virial and hydrodynamic effects. The resulting equilibrium constants indicate that lysozyme does not aggregate significantly as ionic strength increases, even at salt concentrations near the point of salting-out precipitation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. T., Jr, Filmer D. L. Sedimentation equilibrium in reacting systems. IV. Verification of the theory. Biochemistry. 1966 Sep;5(9):2971–2985. doi: 10.1021/bi00873a029. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Timasheff S. N. Preferential interactions of proteins with salts in concentrated solutions. Biochemistry. 1982 Dec 7;21(25):6545–6552. doi: 10.1021/bi00268a034. [DOI] [PubMed] [Google Scholar]
- Banerjee S. K., Pogolotti A., Jr, Rupley J. A. Self-association of lysozyme. Thermochemical measurements: effect of chemical modification of Trp-62, Trp-108, and Glu-35. J Biol Chem. 1975 Oct 25;250(20):8260–8266. [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Williams J. W. Self-association of muramidase (lysozyme) in solution at 25 degrees, pH 7.0, and I = 0.20. Biochemistry. 1970 Oct 27;9(22):4260–4267. doi: 10.1021/bi00824a004. [DOI] [PubMed] [Google Scholar]
- George A., Wilson W. W. Predicting protein crystallization from a dilute solution property. Acta Crystallogr D Biol Crystallogr. 1994 Jul 1;50(Pt 4):361–365. doi: 10.1107/S0907444994001216. [DOI] [PubMed] [Google Scholar]
- Melander W., Horváth C. Salt effect on hydrophobic interactions in precipitation and chromatography of proteins: an interpretation of the lyotropic series. Arch Biochem Biophys. 1977 Sep;183(1):200–215. doi: 10.1016/0003-9861(77)90434-9. [DOI] [PubMed] [Google Scholar]
- Mikol V., Hirsch E., Giegé R. Diagnostic of precipitant for biomacromolecule crystallization by quasi-elastic light-scattering. J Mol Biol. 1990 May 5;213(1):187–195. doi: 10.1016/S0022-2836(05)80130-5. [DOI] [PubMed] [Google Scholar]
- Murphy R. M., Yarmush M. L., Colton C. K. Determining molecular weight distributions of antigen-antibody complexes by quasi-elastic light scattering. Biopolymers. 1991 Oct;31(11):1289–1295. doi: 10.1002/bip.360311107. [DOI] [PubMed] [Google Scholar]
- Nicoli D. F., Benedek G. B. Study of thermal denaturation of lysozyme and other glubular proteins by light-scattering spectroscopy. Biopolymers. 1976 Dec;15(12NA-NA-770103-770104):2421–2437. doi: 10.1002/bip.1976.360151209. [DOI] [PubMed] [Google Scholar]
- Norton R. S., Allerhand A. Participation of tryptophan 62 in the self-association of hen egg white lysozyme. Application of natural abundance carbon 13 nuclear magnetic resonance spectroscopy. J Biol Chem. 1977 Mar 10;252(5):1795–1798. [PubMed] [Google Scholar]
- Riès-Kautt M., Ducruix A., Van Dorsselaer A. Crystallization of previously desalted lysozyme in the presence of sulfate ions. Acta Crystallogr D Biol Crystallogr. 1994 Jul 1;50(Pt 4):366–369. doi: 10.1107/S0907444994001320. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D, Zamora PC, Zukoski CF. Phase behavior of small attractive colloidal particles. Phys Rev Lett. 1996 Jan 1;76(1):150–153. doi: 10.1103/PhysRevLett.76.150. [DOI] [PubMed] [Google Scholar]
- Roth C. M., Neal B. L., Lenhoff A. M. Van der Waals interactions involving proteins. Biophys J. 1996 Feb;70(2):977–987. doi: 10.1016/S0006-3495(96)79641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupley J. A., Butler L., Gerring M., Hartdegen F. J., Pecoraro R. Studies on the enzymic activity of lysozyme, 3. The binding of saccharides. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1088–1095. doi: 10.1073/pnas.57.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., RHODES C. K., HOLCOMB D. N., VAN HOLDE K. E. Physical studies of lysozyme. I. Characterization. J Biol Chem. 1962 Apr;237:1107–1112. [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., VAN HOLDE K. E. Evidence for dimerization of lysozyme in alkaline solution. J Biol Chem. 1961 Dec;236:PC82–PC83. [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., VANHOLDE K. E. PHYSICAL STUDIES OF MURAMIDASE (LYSOZYME). II. PH-DEPENDENT DIMERIZATION. J Biol Chem. 1964 Aug;239:2516–2524. [PubMed] [Google Scholar]
- Shen C. L., Scott G. L., Merchant F., Murphy R. M. Light scattering analysis of fibril growth from the amino-terminal fragment beta(1-28) of beta-amyloid peptide. Biophys J. 1993 Dec;65(6):2383–2395. doi: 10.1016/S0006-3495(93)81312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouri M., Delsanti M., Munch J. P., Lorber B., Giegé R. Dynamic light scattering studies of the aggregation of lysozyme under crystallization conditions. FEBS Lett. 1991 Dec 16;295(1-3):84–88. doi: 10.1016/0014-5793(91)81391-k. [DOI] [PubMed] [Google Scholar]
- Sophianopoulos A. J. Association sites of lysozyme in solution. I. The active site. J Biol Chem. 1969 Jun 25;244(12):3188–3193. [PubMed] [Google Scholar]
- Wang F., Hayter J., Wilson L. J. Salt-induced aggregation of lysozyme studied by cross-linking with glutaraldehyde: implications for crystal growth. Acta Crystallogr D Biol Crystallogr. 1996 Sep 1;52(Pt 5):901–908. doi: 10.1107/S0907444996005227. [DOI] [PubMed] [Google Scholar]
- Wills P. R., Nichol L. W., Siezen R. J. The indefinite self-association of lysozyme: consideration of composition-dependent activity coefficients. Biophys Chem. 1980 Feb;11(1):71–82. doi: 10.1016/0301-4622(80)85009-5. [DOI] [PubMed] [Google Scholar]
- Wilson L. J., Adcock-Downey L., Pusey M. L. Monomer concentrations and dimerization constants in crystallizing lysozyme solutions by dialysis kinetics. Biophys J. 1996 Oct;71(4):2123–2129. doi: 10.1016/S0006-3495(96)79412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


