Abstract
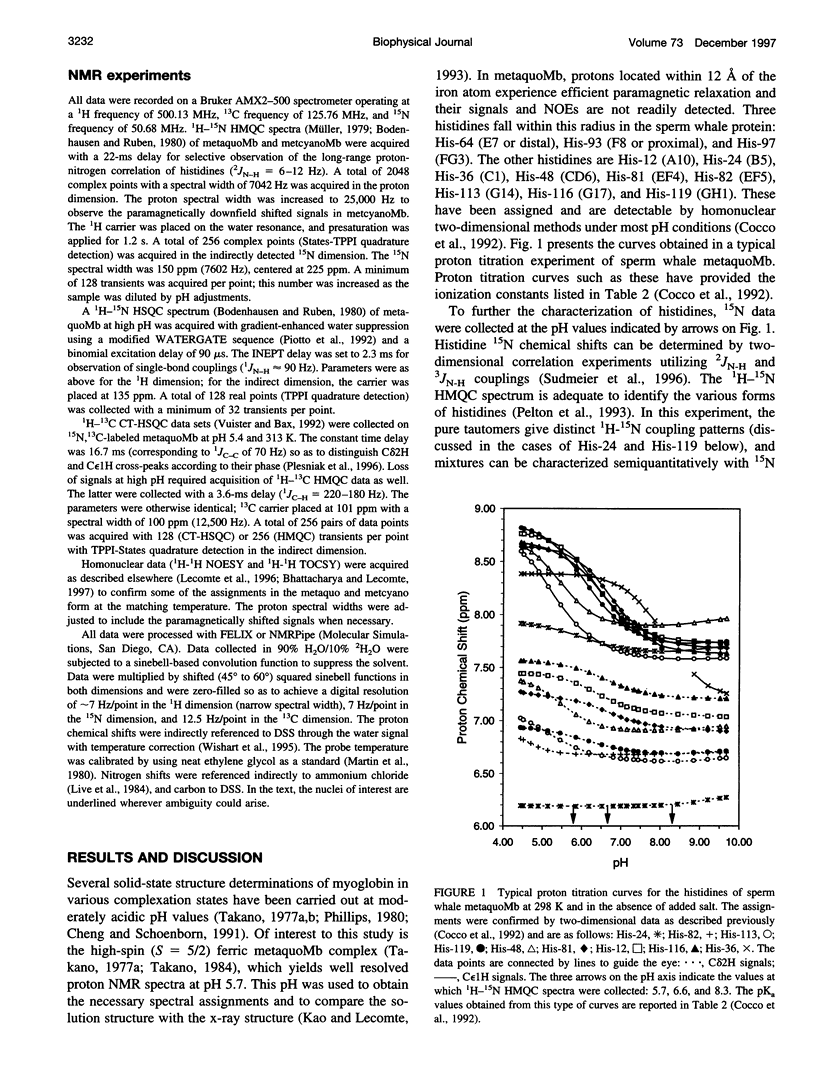
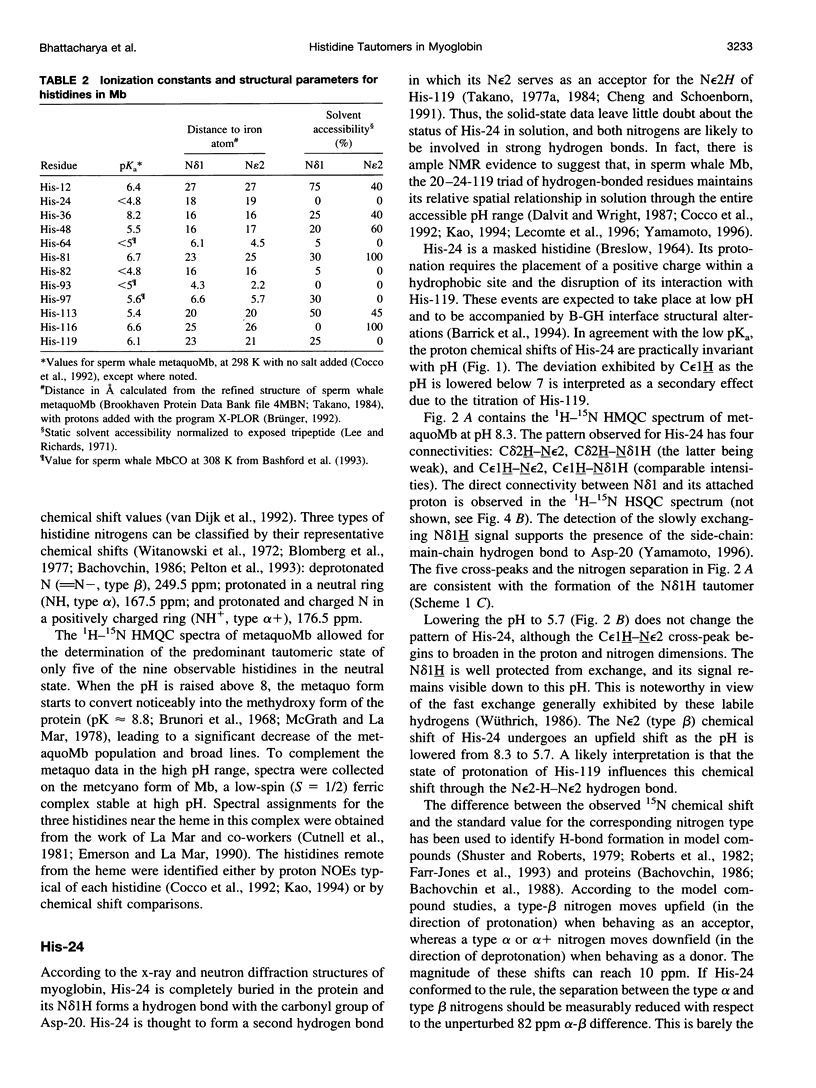
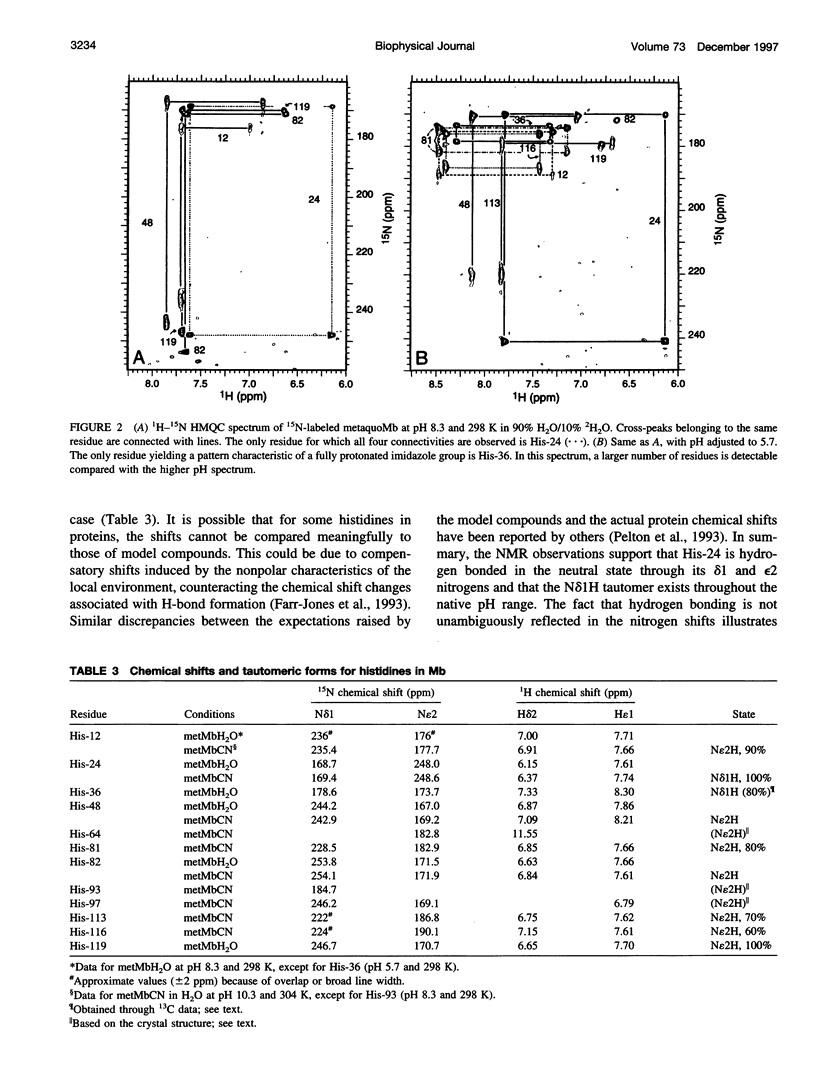
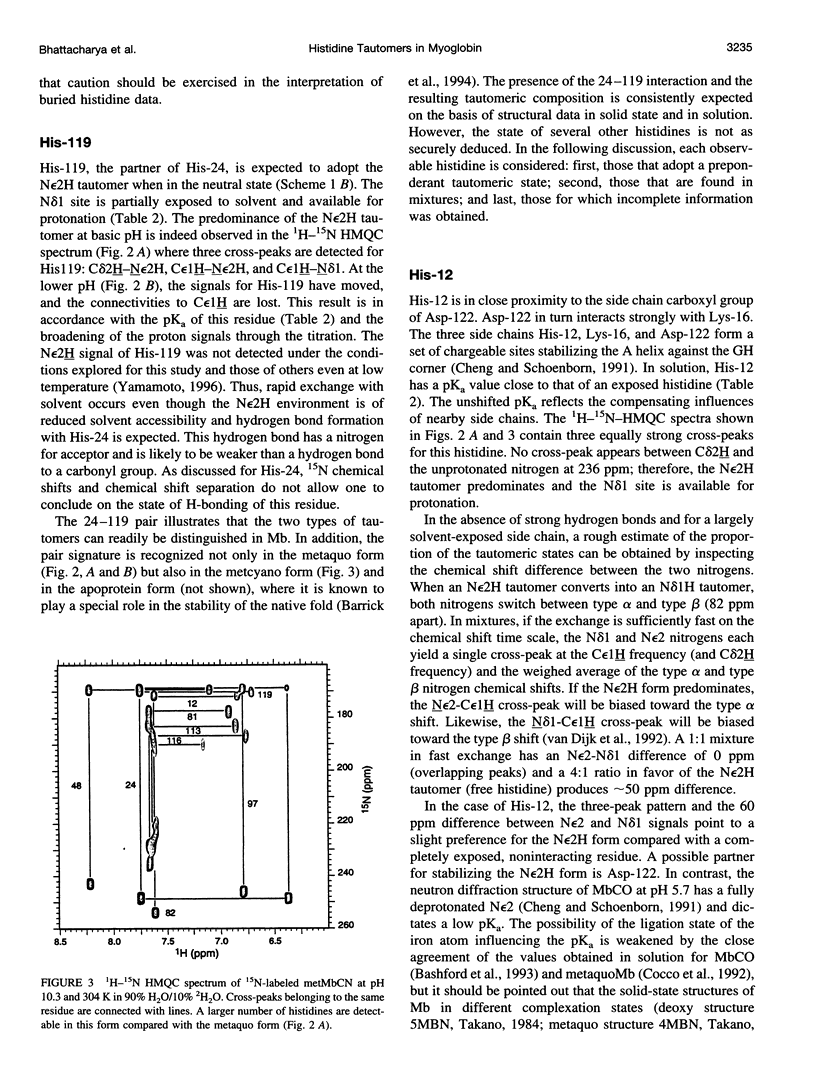
1H-15N HMQC spectra were collected on 15N-labeled sperm whale myoglobin (Mb) to determine the tautomeric state of its histidines in the neutral form. By analyzing metaquoMb and metcyanoMb data sets collected at various pH values, cross-peaks were assigned to the imidazole rings and their patterns interpreted. Of the nine histidines not interacting with the heme in sperm whale myoglobin, it was found that seven (His-12, His-48, His-81, His-82, His-113, His-116, and His-119) are predominantly in the N epsilon2H form with varying degrees of contribution from the Ndelta1 H form. The eighth, His-24, is in the Ndelta1H state as expected from the solid state structure. 13C correlation spectra were collected to probe the state of the ninth residue (His-36). Tentative interpretation of the data through comparison with horse Mb suggested that this ring is predominantly in the Ndelta1H state. In addition, signals were observed from the histidines associated with the heme (His-64, His-93, and His-97) in the 1H-15N HMQC spectra of the metcyano form. In several cases, the tautomeric state of the imidazole ring could not be derived from inspection of the solid state structure. It was noted that hydrogen bonding of the ring was not unambiguously reflected in the nitrogen chemical shift. With the experimentally determined tautomeric state composition in solution, it will be possible to broaden the scope of other studies focused on the electrostatic contribution of histidines to the thermodynamic properties of myoglobin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRESLOW E. CHANGES IN SIDE CHAIN REACTIVITY ACCOMPANYING THE BINDING OF HEME TO SPERM WHALE APOMYOGLOBIN. J Biol Chem. 1964 Feb;239:486–496. [PubMed] [Google Scholar]
- Bachovchin W. W. 15N NMR spectroscopy of hydrogen-bonding interactions in the active site of serine proteases: evidence for a moving histidine mechanism. Biochemistry. 1986 Nov 18;25(23):7751–7759. doi: 10.1021/bi00371a070. [DOI] [PubMed] [Google Scholar]
- Bachovchin W. W., Wong W. Y., Farr-Jones S., Shenvi A. B., Kettner C. A. Nitrogen-15 NMR spectroscopy of the catalytic-triad histidine of a serine protease in peptide boronic acid inhibitor complexes. Biochemistry. 1988 Oct 4;27(20):7689–7697. doi: 10.1021/bi00420a018. [DOI] [PubMed] [Google Scholar]
- Banci L., Bertini I., Turano P., Tien M., Kirk T. K. Proton NMR investigation into the basis for the relatively high redox potential of lignin peroxidase. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6956–6960. doi: 10.1073/pnas.88.16.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick D., Hughson F. M., Baldwin R. L. Molecular mechanisms of acid denaturation. The role of histidine residues in the partial unfolding of apomyoglobin. J Mol Biol. 1994 Apr 15;237(5):588–601. doi: 10.1006/jmbi.1994.1257. [DOI] [PubMed] [Google Scholar]
- Bashford D., Case D. A., Dalvit C., Tennant L., Wright P. E. Electrostatic calculations of side-chain pK(a) values in myoglobin and comparison with NMR data for histidines. Biochemistry. 1993 Aug 10;32(31):8045–8056. doi: 10.1021/bi00082a027. [DOI] [PubMed] [Google Scholar]
- Beck von Bodman S., Schuler M. A., Jollie D. R., Sligar S. G. Synthesis, bacterial expression, and mutagenesis of the gene coding for mammalian cytochrome b5. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9443–9447. doi: 10.1073/pnas.83.24.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
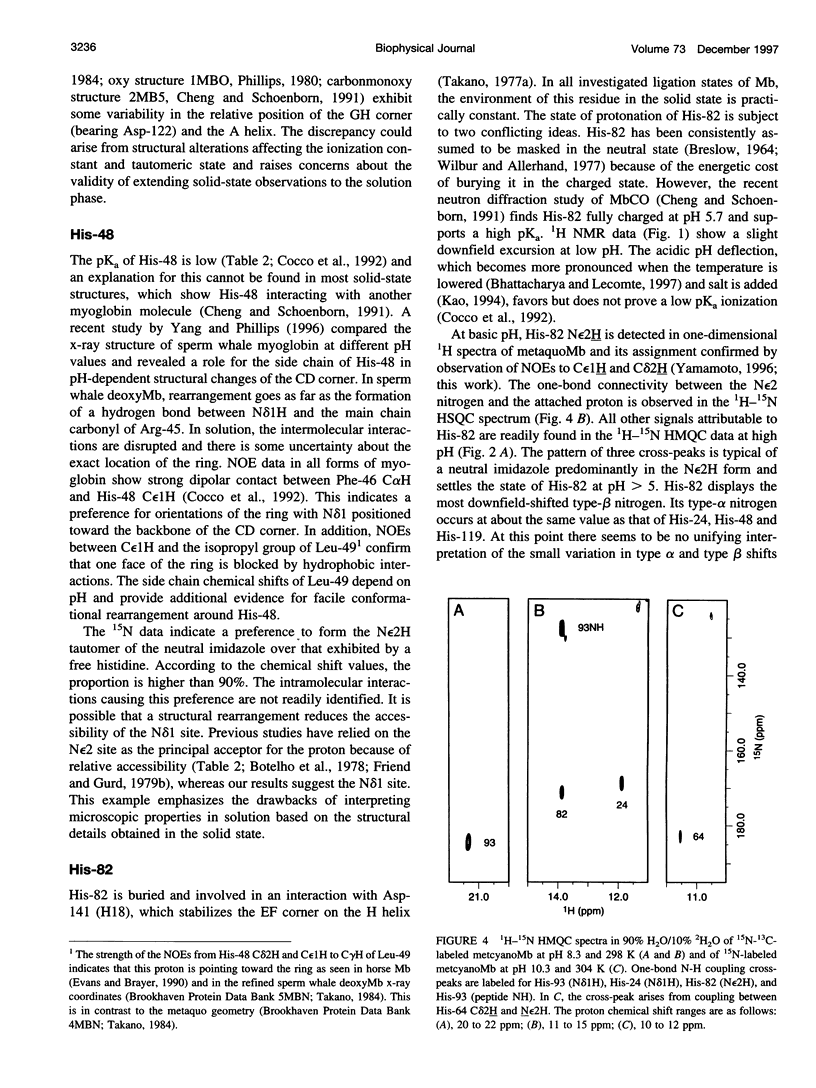
- Bhattacharya S., Lecomte J. T. Temperature dependence of histidine ionization constants in myoglobin. Biophys J. 1997 Dec;73(6):3241–3256. doi: 10.1016/S0006-3495(97)78349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg F., Maurer W., Rüterjans H. Nuclear magnetic resonance investigation of 15N-labeled histidine in aqueous solution. J Am Chem Soc. 1977 Dec 7;99(25):8149–8159. doi: 10.1021/ja00467a005. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., Friend S. H., Matthew J. B., Lehman L. D., Hanania G. I., Gurd F. R. Proton nuclear magnetic resonance study of histidine ionizations in myoglobins of various species. Comparison of observed and computed pK values. Biochemistry. 1978 Nov 28;17(24):5197–5205. doi: 10.1021/bi00617a020. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., Gurd F. R. Proton nuclear magnetic resonance study of histidine ionizations in myoglobins of various species. Specific assignment of individual resonances. Biochemistry. 1978 Nov 28;17(24):5188–5196. doi: 10.1021/bi00617a019. [DOI] [PubMed] [Google Scholar]
- Brunori M., Amiconi G., Antonin E., Wyman J., Zito R., Fanelli A. R. The transition between 'acid' and 'alkaline' ferric heme proteins. Biochim Biophys Acta. 1968 Feb 19;154(2):315–322. doi: 10.1016/0005-2795(68)90045-7. [DOI] [PubMed] [Google Scholar]
- Cheng X. D., Schoenborn B. P. Neutron diffraction study of carbonmonoxymyoglobin. J Mol Biol. 1991 Jul 20;220(2):381–399. doi: 10.1016/0022-2836(91)90020-7. [DOI] [PubMed] [Google Scholar]
- Cocco M. J., Kao Y. H., Phillips A. T., Lecomte J. T. Structural comparison of apomyoglobin and metaquomyoglobin: pH titration of histidines by NMR spectroscopy. Biochemistry. 1992 Jul 21;31(28):6481–6491. doi: 10.1021/bi00143a018. [DOI] [PubMed] [Google Scholar]
- Dalvit C., Wright P. E. Assignment of resonances in the 1H nuclear magnetic resonance spectrum of the carbon monoxide complex of sperm whale myoglobin by phase-sensitive two-dimensional techniques. J Mol Biol. 1987 Mar 20;194(2):313–327. doi: 10.1016/0022-2836(87)90378-0. [DOI] [PubMed] [Google Scholar]
- Emerson S. D., La Mar G. Solution structural characteristics of cyanometmyoglobin: resonance assignment of heme cavity residues by two-dimensional NMR. Biochemistry. 1990 Feb 13;29(6):1545–1556. doi: 10.1021/bi00458a028. [DOI] [PubMed] [Google Scholar]
- Evans S. V., Brayer G. D. High-resolution study of the three-dimensional structure of horse heart metmyoglobin. J Mol Biol. 1990 Jun 20;213(4):885–897. doi: 10.1016/S0022-2836(05)80270-0. [DOI] [PubMed] [Google Scholar]
- Finzel B. C., Poulos T. L., Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-A resolution. J Biol Chem. 1984 Nov 10;259(21):13027–13036. [PubMed] [Google Scholar]
- Friend S. H., Gurd F. R. Electrostatic stabilization in myoglobin. Interactive free energies between individual sites. Biochemistry. 1979 Oct 16;18(21):4620–4630. doi: 10.1021/bi00588a024. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Gurd F. R. Electrostatic stabilization in myoglobin. pH dependence of summed electrostatic contributions. Biochemistry. 1979 Oct 16;18(21):4612–4619. doi: 10.1021/bi00588a023. [DOI] [PubMed] [Google Scholar]
- Garcia-Moreno B., Chen L. X., March K. L., Gurd R. S., Gurd F. R. Electrostatic interactions in sperm whale myoglobin. Site specificity, roles in structural elements, and external electrostatic potential distributions. J Biol Chem. 1985 Nov 15;260(26):14070–14082. [PubMed] [Google Scholar]
- Garrett D. S., Seok Y. J., Liao D. I., Peterkofsky A., Gronenborn A. M., Clore G. M. Solution structure of the 30 kDa N-terminal domain of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system by multidimensional NMR. Biochemistry. 1997 Mar 4;36(9):2517–2530. doi: 10.1021/bi962924y. [DOI] [PubMed] [Google Scholar]
- Garrett D. S., Seok Y. J., Peterkofsky A., Clore G. M., Gronenborn A. M. Identification by NMR of the binding surface for the histidine-containing phosphocarrier protein HPr on the N-terminal domain of enzyme I of the Escherichia coli phosphotransferase system. Biochemistry. 1997 Apr 15;36(15):4393–4398. doi: 10.1021/bi970221q. [DOI] [PubMed] [Google Scholar]
- Lecomte J. T., Cocco M. J. Structural features of the protoporphyrin-apomyoglobin complex: a proton NMR spectroscopy study. Biochemistry. 1990 Dec 18;29(50):11057–11067. doi: 10.1021/bi00502a007. [DOI] [PubMed] [Google Scholar]
- Lecomte J. T., Kao Y. H., Cocco M. J. The native state of apomyoglobin described by proton NMR spectroscopy: the A-B-G-H interface of wild-type sperm whale apomyoglobin. Proteins. 1996 Jul;25(3):267–285. doi: 10.1002/(SICI)1097-0134(199607)25:3<267::AID-PROT1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Loewenthal R., Sancho J., Fersht A. R. Histidine-aromatic interactions in barnase. Elevation of histidine pKa and contribution to protein stability. J Mol Biol. 1992 Apr 5;224(3):759–770. doi: 10.1016/0022-2836(92)90560-7. [DOI] [PubMed] [Google Scholar]
- McGrath T. M., La Mar G. N. Proton NMR study of the thermodynamics and kinetics of the acid in equilibrium base transitions in reconstituted metmyoglobins. Biochim Biophys Acta. 1978 May 24;534(1):99–111. doi: 10.1016/0005-2795(78)90480-4. [DOI] [PubMed] [Google Scholar]
- Morikis D., Champion P. M., Springer B. A., Sligar S. G. Resonance raman investigations of site-directed mutants of myoglobin: effects of distal histidine replacement. Biochemistry. 1989 May 30;28(11):4791–4800. doi: 10.1021/bi00437a041. [DOI] [PubMed] [Google Scholar]
- Oh B. H., Markley J. L. Multinuclear magnetic resonance studies of the 2Fe.2S* ferredoxin from Anabaena species strain PCC 7120. 3. Detection and characterization of hyperfine-shifted nitrogen-15 and hydrogen-1 resonances of the oxidized form. Biochemistry. 1990 Apr 24;29(16):4012–4017. doi: 10.1021/bi00468a031. [DOI] [PubMed] [Google Scholar]
- Pelton J. G., Torchia D. A., Meadow N. D., Roseman S. Tautomeric states of the active-site histidines of phosphorylated and unphosphorylated IIIGlc, a signal-transducing protein from Escherichia coli, using two-dimensional heteronuclear NMR techniques. Protein Sci. 1993 Apr;2(4):543–558. doi: 10.1002/pro.5560020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Piotto M., Saudek V., Sklenár V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR. 1992 Nov;2(6):661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Plesniak L. A., Connelly G. P., Wakarchuk W. W., McIntosh L. P. Characterization of a buried neutral histidine residue in Bacillus circulans xylanase: NMR assignments, pH titration, and hydrogen exchange. Protein Sci. 1996 Nov;5(11):2319–2328. doi: 10.1002/pro.5560051118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos T. L., Edwards S. L., Wariishi H., Gold M. H. Crystallographic refinement of lignin peroxidase at 2 A. J Biol Chem. 1993 Feb 25;268(6):4429–4440. doi: 10.2210/pdb1lga/pdb. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. A hypothetical model of the cytochrome c peroxidase . cytochrome c electron transfer complex. J Biol Chem. 1980 Nov 10;255(21):10322–10330. [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. The stereochemistry of peroxidase catalysis. J Biol Chem. 1980 Sep 10;255(17):8199–8205. [PubMed] [Google Scholar]
- Reynolds W. F., Peat I. R., Freedman M. H., Lyerla J. R., Jr Determination of the tautomeric form of the imidazole ring of L-histidine in basic solution by carbon-13 magnetic resonance spectroscopy. J Am Chem Soc. 1973 Jan 24;95(2):328–331. doi: 10.1021/ja00783a006. [DOI] [PubMed] [Google Scholar]
- Shire S. J., Hanania G. I., Gurd F. R. Electrostatic effects in myoglobin. Hydrogen ion equilibria in sperm whale ferrimyoglobin. Biochemistry. 1974 Jul 2;13(14):2967–2974. doi: 10.1021/bi00711a028. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Farr-Jones S., Griffin R. G., Bachovchin W. W. Crystal versus solution structures of enzymes: NMR spectroscopy of a crystalline serine protease. Science. 1989 May 26;244(4907):961–964. doi: 10.1126/science.2499045. [DOI] [PubMed] [Google Scholar]
- Sudmeier J. L., Ash E. L., Günther U. L., Luo X., Bullock P. A., Bachovchin W. W. HCN, a triple-resonance NMR technique for selective observation of histidine and tryptophan side chains in 13C/15N-labeled proteins. J Magn Reson B. 1996 Dec;113(3):236–247. doi: 10.1006/jmrb.1996.0182. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Tanokura M. 1H-NMR study on the tautomerism of the imidazole ring of histidine residues. I. Microscopic pK values and molar ratios of tautomers in histidine-containing peptides. Biochim Biophys Acta. 1983 Feb 15;742(3):576–585. doi: 10.1016/0167-4838(83)90276-5. [DOI] [PubMed] [Google Scholar]
- Tolman J. R., Flanagan J. M., Kennedy M. A., Prestegard J. H. Nuclear magnetic dipole interactions in field-oriented proteins: information for structure determination in solution. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9279–9283. doi: 10.1073/pnas.92.20.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk A. A., Scheek R. M., Dijkstra K., Wolters G. K., Robillard G. T. Characterization of the protonation and hydrogen bonding state of the histidine residues in IIAmtl, a domain of the phosphoenolpyruvate-dependent mannitol-specific transport protein. Biochemistry. 1992 Sep 22;31(37):9063–9072. doi: 10.1021/bi00152a050. [DOI] [PubMed] [Google Scholar]
- Wasylishen R. E., Tomlinson G. Application of long-range 13C,H nuclear spin-spin coupling constants in the study of imidazole tautomerism in L-histidine, and related compounds. Can J Biochem. 1977 May;55(5):579–582. doi: 10.1139/o77-083. [DOI] [PubMed] [Google Scholar]
- Wasylishen R. E., Tomlinson G. pH-dependence of 13C chemical shifts and 13C,H coupling constants in imidazole and L-histidine. Biochem J. 1975 Jun;147(3):605–607. doi: 10.1042/bj1470605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur D. J., Allerhand A. Titration behavior and tautomeric states of individual histidine residues of myoglobins. Application of natural abundance carbon 13 nuclear magnetic resonance spectroscopy. J Biol Chem. 1977 Jul 25;252(14):4968–4975. [PubMed] [Google Scholar]
- Wishart D. S., Bigam C. G., Yao J., Abildgaard F., Dyson H. J., Oldfield E., Markley J. L., Sykes B. D. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR. 1995 Sep;6(2):135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- Xia B., Cheng H., Skjeldal L., Coghlan V. M., Vickery L. E., Markley J. L. Multinuclear magnetic resonance and mutagenesis studies of the histidine residues of human mitochondrial ferredoxin. Biochemistry. 1995 Jan 10;34(1):180–187. doi: 10.1021/bi00001a022. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. H NMR probes for inter-segmental hydrogen bonds in myoglobins. J Biochem. 1996 Jul;120(1):126–132. doi: 10.1093/oxfordjournals.jbchem.a021373. [DOI] [PubMed] [Google Scholar]
- Yang A. S., Honig B. Structural origins of pH and ionic strength effects on protein stability. Acid denaturation of sperm whale apomyoglobin. J Mol Biol. 1994 Apr 15;237(5):602–614. doi: 10.1006/jmbi.1994.1258. [DOI] [PubMed] [Google Scholar]
- Yang F., Phillips G. N., Jr Crystal structures of CO-, deoxy- and met-myoglobins at various pH values. J Mol Biol. 1996 Mar 8;256(4):762–774. doi: 10.1006/jmbi.1996.0123. [DOI] [PubMed] [Google Scholar]
- Yu L. P., Smith G. M. Characterization of pH-dependent conformational heterogeneity in Rhodospirillum rubrum cytochrome c2 using 15N and 1H NMR. Biochemistry. 1990 Mar 27;29(12):2920–2925. doi: 10.1021/bi00464a005. [DOI] [PubMed] [Google Scholar]
- van Dijk A. A., de Lange L. C., Bachovchin W. W., Robillard G. T. Effect of phosphorylation on hydrogen-bonding interactions of the active site histidine of the phosphocarrier protein HPr of the phosphoenolpyruvate-dependent phosphotransferase system determined by 15N NMR spectroscopy. Biochemistry. 1990 Sep 4;29(35):8164–8171. doi: 10.1021/bi00487a026. [DOI] [PubMed] [Google Scholar]


