Abstract
This article reports on the application of organically modified silica (ORMOSIL) nanoparticles as a nonviral vector for efficient in vivo gene delivery. Highly monodispersed, stable aqueous suspension of nanoparticles, surface-functionalized with amino groups for binding of DNA, were prepared and characterized. Stereotaxic injections of nanoparticles, complexed with plasmid DNA encoding for EGFP, into the mouse ventral midbrain and into lateral ventricle, allowed us to fluorescently visualize the extensive transfection of neuronal-like cells in substantia nigra and areas surrounding the lateral ventricle. No ORMOSIL-based toxicity was observed 4 weeks after transfection. The efficiency of transfection equaled or exceeded that obtained in studies using a viral vector. An in vivo optical imaging technique (a fiber-based confocal fluorescent imaging system) provided an effective means to show the retention of viability of the transfected cells. The ORMOSIL-mediated transfections also were used to manipulate the biology of the neural stem/progenitor cells in vivo. Transfection of a plasmid expressing the nucleus-targeting fibroblast growth factor receptor type 1 resulted in significant inhibition of the in vivo incorporation of bromodeoxyuridine into the DNA of the cells in the subventricular zone and the adjacent rostral migratory stream. This in vivo approach shows that the nuclear receptor can control the proliferation of the stem/progenitor cells in this region of the brain. The results of this nanomedicine approach using ORMOSIL nanoparticles as a nonviral gene delivery platform have a promising future direction for effective therapeutic manipulation of the neural stem/progenitor cells as well as in vivo targeted brain therapy.
Keywords: gene transfection, neurological, stem, progenitor cells
The application of nanotechnology to biomedical research is expected to have a major impact leading to the development of new types of diagnostic and therapeutic tools (1, 2). One focus in nanobiotechnology is the development and use of nonviral vectors for safe and efficient gene delivery (3). The potential for the treatment of genetic disorders has advanced tremendously with the ability to identify specific genes whose defect or absence is responsible for a particular pathological condition. In genetically based diseases, specifically designed therapeutic genes, if successfully delivered into the appropriate cells, will provide a significant advancement in disease therapy. Therefore, in vivo gene delivery is an area of considerable interest where genetic materials (e.g., DNA, RNA, and oligonucleotides) could be used to inhibit undesirable gene expression or to synthesize therapeutic proteins (4, 5). Given the potential of gene transfer as a therapeutic tool, the application of nanotechnology in the development of nonviral transfection vectors for gene therapy is urgently needed.
For effective gene therapy, a genetic payload must be delivered to the targeted cell/tissue, then enter such cell and be transported to the nucleus to achieve expression. The initiating event, which is the entrance of the genetic material into the cell without the use of a viral vector, is limited because of the need to supply the DNA to the surface of the cell in sufficient concentration to effect entrance (6). Ultrafine silica nanoparticles, functionalized with amino groups, have been shown to bind and protect plasmid DNA from enzymatic digestion and to effect cell transfection in vitro (7–9). Recent work by our group has established the feasibility of using amino-functionalized organically modified silica (ORMOSIL) nanoparticles as a nonviral vector for in vitro gene transfection (10). This formulation of nanoparticles overcomes many of the limitations of “unmodified” silica nanoparticles. The presence of both hydrophobic and hydrophilic groups on the precursor alkoxy organosilane helps them to self-assemble as both normal micelles and reverse micelles under appropriate conditions. ORMOSIL nanoparticles are prepared from oil-in-water microemulsions, avoiding corrosive solvents such as cyclohexane, and through a complex purification process. Their organic groups can be further modified for the attachment of targeting molecules and can be possibly biodegraded through the biochemical decomposition of the silicon–carbon bond (11, 12). The presence of this organic group also imparts some degree of flexibility to the otherwise rigid silica matrix, which enhances the stability of such particles in aqueous systems.
Gene therapy for cerebral and cerebrovascular diseases will become clinically relevant upon the development of effective and safe gene-transfer vectors (13). Viral gene-transfer techniques have aroused increasing interest in the treatment of traumatic brain injury/ischemia, neurodegenerative, developmental, and neoplastic brain disorders. These vectors have the ability to deliver a specific gene to the nucleus of a cell and have it expressed through its integration into the genome or as episomal vectors (5). A critical concern for any gene therapy is the safety of the vector. It has been recognized that there is a risk of excessive immune response (adenovirus) as well as insertional mutagenesis (retroviruses) when viruses are used as transfection vectors. Actual deaths have occurred in human trials, leading to a halt in further use of viral vectors for gene transfection, which has been a major setback for this area of research (14, 15). Although these viral vectors are attractive in terms of the scientific strategy, such systems suffer from inherent difficulties in pharmaceutical processing, scale-up, immunogenicity, and reversion of an engineered virus (4).
Safe and effective nanotechnology-based gene delivery vectors could potentially be used to counteract brain pathological processes, augment compensatory mechanisms, and influence the brain regenerative capacities. In mature mammalian brain, certain areas retain the capacity for neurogenesis (16). One such area is the subventricular zone (SVZ) of the lateral ventricle (LV). The SVZ contains a population of slowly dividing stem cells that generate faster-proliferating (“transit-amplifying”) neural progenitor cells. The progenitor cells give rise to brain neurons, astrocytes, and oligodendrocytes once removed from this proliferating state (17–19). The SVZ population of multipotent neural stem/progenitor cells (NSPCs) expands when treated in vitro with exogenous FGF2. The intracerebroventricular administration of FGF2 peptide or FGF2-expressing adenovirus evokes a significant incorporation of BrdUrd by the SVZ cells, suggesting that stimulation of FGF receptors by exogenous FGFs could influence the proliferation of the NSPC cells in vivo (20). However, although secreted members of the FGF family may act as mitogens through receptors on the cell surface, others, including the nuclear localization signal (NLS)-containing forms of FGF2, may produce their biological effects in the nucleus. All cells isolated from the SVZ were found to express the FGF receptor type 1 (FGFR1) (21). In proliferating human neural progenitor cells, FGFR1 is associated with the cytoplasm and plasma membrane (22, 23). However, a newly synthesized FGFR1 also is released from the endoplasmic reticulum into the cytosol by a process enabled by a FGFR1 transmembrane domain and is then transported into the nucleus by importin-β (22–24). Nuclear accumulation of FGFR1 accompanies differentiation of the neural progenitor cells, induced by bone morphogenetic protein 7, cAMP, or other stimuli, and is essential as well as sufficient for inducing cell differentiation (22, 23). A compelling question is whether FGFR1 may have a similar function in vivo. In the present study, we have transfected the mouse brain SVZ cells in vivo with the nucleus-targeting FGFR1(SP-/NLS) to determine the role of nuclear receptor in the biology of these cells.
In this article, we report the successful in vivo transfection and modulation of the activity of neural cells in the brains of mice by using ORMOSIL nanoparticles. Our goal was to verify whether ORMOSIL-mediated DNA transfer may effectively transfect the SVZ cells and thus serve as a tool to elucidate the in situ mechanisms that govern these critically important cells and to manipulate their biology for therapeutic purposes. The present study demonstrates the potential of ORMOSIL nanoparticle technology to effect gene transfection, resulting in the modulation of the SVZ cell function in vivo.
Materials and Methods
Materials. Plasmid expressing EGFP with the cytomegalovirus early promoter (pEGFP-N2) and mAb to EGFP were purchased from Clontech. Plasmids were used to transform Escherichia coli, which were grown, and the plasmids were isolated by using an endotoxin-free kit (Qiagen, Valencia, CA). Polyclonal rabbit anti-tyrosine hydroxylase (TH) Ab and BrdUrd were obtained from Sigma, and rabbit anti-C-terminal FGFR1 Ab was obtained from Stratagene. Anti-BrdUrd mAb and Alexa Fluor 488-conjugated goat anti-mouse IgG were obtained from Molecular Probes. Cy3-conjugated goat anti-rabbit IgG was purchased from Jackson ImmunoResearch. Normal goat serum (NGS) was obtained from ImmunoGen (Cambridge, MA).
Synthesis of ORMOSIL/pEGFP-N2 Nanoparticles. The general procedure for the preparation and characterization of ORMOSIL/DNA nanoparticles was described in refs. 10 and 25 and is briefly outlined as follows: Typically, the micelles were prepared by dissolving a fixed amount of the surfactant (aerosol OT, Sigma) and 1-butanol in doubly distilled water. Neat triethoxyvinylsilane was added to this micellar system, and the resulting solution was stirred for ≈30 min. Amino-functionalized ORMOSIL nanoparticles were then prepared by precipitation with 3-aminopropyltriethoxysilane, resulting in the incorporation of cationic amino groups on the surface of the ORMOSIL nanoparticles. The surfactants were removed by dialysis, and elemental analysis and morphology of the nanoparticles was determined by using x-ray photoelectron spectrometry and transmission electron microscopy (10). DNA loading of ORMOSIL nanoparticles was accomplished by incubation with pEGFP-N2 for 30 min at room temperature. The resulting ORMOSIL/pEGFP-N2 nanoparticles were suspended in phosphate-buffered saline (PBS; 135 μg/ml DNA) and filter-sterilized before injection.
In Vivo Transfection. The pEGFP-N2 (DNA control), ORMOSIL (nanoparticle control), and ORMOSIL/pEGFP-N2 were injected into adult mice of both sexes by using stereotaxic surgery with equivalent concentrations of control injected materials and ORMOSIL/plasmid (26, 27). Mice were anesthetized; an incision into the dorsal aspect of the head was made, exposing the cranium and the bregma, and a fine dental air-drill was used to drill a hole in the skull. Slow microinjection was used to deliver nanoparticles (2–6 μl containing 0.03–0.08 μg of plasmid DNA) into substantia nigra (SN) or the brain LV. Seven or 10 days after injection, mice were deeply anesthetized and perfused transcardially with PBS, followed by 4% paraformaldehyde to fix the brain tissue. The brains were removed and frozen, and 20-μm coronal or sagittal cryostat-cut sections were prepared and processed for immunocytochemistry for detection of expression of EGFP.
Immunocytochemistry. The fixed, free-floating brain sections were incubated in 10% NGS, followed by mouse anti-EGFP mAb (1:100 in 10% NGS) or in combination with polyclonal rabbit anti-TH Ab (1:1,000 in 10% NGS) for 72 h. After multiple rinses in PBS, sections were incubated for 2–3 h with a mixture of the appropriate secondary Abs (Alexa Fluor 488-conjugated goat anti-mouse IgG; 1:150 in 10% NGS or in combination with Cy3-conjugated goat anti-rabbit IgG; 1:600 in 10% NGS). EGFP expression was visualized by using standard fluorescence microscopy. Double immunostaining for EGFP and TH was determined from confocal images obtained in a sequential mode by using a confocal microscope (MRC 1024, Bio-Rad).
Monitoring Transfected Cells in Vivo. Mice transfected with ORMOSIL/pEGFP-N2 were subjected to the second stereotaxic surgery, and transfected cells were visualized in live animals by using a fibered confocal fluorescent microscopy (Cell-viZio, Discovery Technology International, Sarasota, FL). The Cell-viZio system uses a miniature fiber-optic probe (350-μm tip) that can be directly inserted into the brain tissue, permitting confocal imaging with a cellular resolution of 2.5 μm. The excitation light source is a 488-nm Argon ion laser line (Coherent, Santa Clara, CA), which is coupled and then focused, in sequence, through each individual microfiber. The probe was attached to a stereotaxic frame and gradually lowered into the ventricle through a 1-mm opening in the skull. The beveled tip of the probe allowed penetration in soft tissue without the need for a cannula. The resulting emitted fluorescence light, after filtering (500–650 nm), is detected by the detector housed in the main unit. The image is then reconstructed and shown on a real-time display at 12 frames per second.
FGFR1(SP-/NLS) Transfection and BrdUrd Incorporation. The pcDNA3.1 plasmid expressing FGFR1 with the signal peptide replaced with NLS was constructed as described in ref. 28. The ORMOSIL/pFGFR1(SP-/NLS) nanoparticle complex (6 μl containing 0.08 μg of DNA) was injected into the left LV. Seven days after injection, mice were injected intraperitoneally with BrdUrd (100 mg/kg) and perfused with 4% paraformaldehyde 5 h later as described above. Consecutive sagittal brain sections, encompassing both LVs, were incubated in 4% paraformaldehyde, washed with PBS, treated with 0.5% Triton X-100, and washed again with PBS. The sections were then treated with 2 M HCl at 37°C for 15 min, neutralized in alkaline PBS (pH 8.5), washed with PBS (pH 7.4), and incubated with anti-BrdUrd mAb (1:200 in 10% NGS), followed by goat anti-mouse-Alexa Fluor 488 secondary Ab (1:150 in 10% NGS). The expression of FGFR1 in fixed sections was determined fluorescently after incubation with FGFR1 McAb6, followed by goat anti-mouse-Alexa Fluor 488 secondary Ab (29).
Results and Discussion
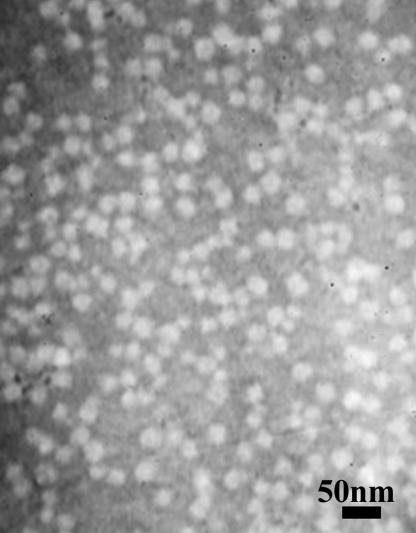
Chemical and structural analyses of the ORMOSIL nanoparticles were performed by using x-ray photoelectron spectrometry and transmission electron microscopy. The chemical analysis confirmed the presence of nitrogen groups in the ORMOSIL nanoparticle preparation. The relative percentages of carbon, oxygen, nitrogen, and silicon were found to be 54.3 ± 0.8, 29.5 ± 0.7, 2.1 ± 0.4, and 12.7 ± 1.5, respectively. The presence of the organic group reduces the overall rigidity and density of the particle, which enhances the stability of such particles in aqueous systems and protects against precipitation. Optimal loading of the plasmid (pEGFP-N2) was determined to be 135 μg of DNA per ≈1014 nanoparticles. Fig. 1 represents the transmission electron microscopy images of ORMOSIL/pEGFP-N2 nanoparticles. The plasmid-loaded nanoparticles retained their monodispersion and exhibit a morphology similar to that previously shown for free ORMOSIL nanoparticles (10).
Fig. 1.
Transmission electron micrograph of ORMOSIL/pEGFP-N2 nanoparticles.
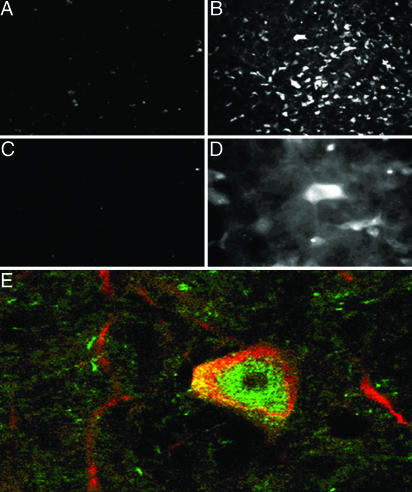
ORMOSIL nanoparticles, ORMOSIL/pEGFP-N2 nanocomplexes, and pEGFP-N2 plasmids were injected directly into the brain tissue and were examined as vehicle for gene transfer directly into the SN pars compacta (SNc), an area richly populated with neuronal cells. The SNc region is of considerable interest for gene therapy, given its role in Parkinson's disease and several psychiatric disorders (30–32). Initially, we attempted to evaluate the EGFP expression by observing its fluorescence directly. However, the fixation process, necessary for preserving the brain tissue cellular structure, resulted in reduced EGFP fluorescence. Therefore, the presence of EGFP expression was determined by using indirect immunofluorescence with antibodies to EGFP. After the injection of the ORMOSIL nanoparticles, a few SNc cells exhibited a weak autofluorescence with no detectable EGFP immunoreactivity (Fig. 2A). Similar results were seen when free plasmid was injected. In contrast, injection of OROMSIL/pEGFP-N2 resulted in a robust EGFP expression in neuron-shaped cells (Fig. 2B), which was not observed in the absence of the primary anti-EGFP Ab (Fig. 2C). Fig. 2D shows the clear neuronal morphology of EGFP-immunopositive cells. The majority of the SNc neurons are dopaminergic and can be specifically detected with Ab against TH (33). Double immunostaining with anti-TH and anti-EGFP mAb revealed that the majority of TH-expressing cells were immunopositive for EGFP. TH immunostaining (red) was observed in peripheral cytoplasm and axonal-like processes, whereas EGFP immunoreactivity (green) was concentrated in the central cell area (Fig. 2E). The efficiency of ORMOSIL-mediated gene transfer was comparable with the most effective ICP4(-) herpes simplex virus 1 vector previously used in our laboratory (27) and was higher than that seen with herpes simplex virus 1 amplicon vector (27).
Fig. 2.
ORMOSIL nanoparticle transfection in the SNc. (A) DNA-free ORMOSIL injection showing no substantial immunostaining for EGFP. (B–E) Injection of ORMOSIL-pEGFP-N2 complex into SNc. (B) Multiple cells with typical dopaminergic neuron morphology are immunostained positive for EGFP. (C) No immunostaining is observed without primary anti-EGFP Ab. (D) EGFP immunostaining of neuron-shaped cells (higher magnification). (E) Transfected EGFP (green) is expressed in TH-immunopositive (red) dopaminergic neuron.
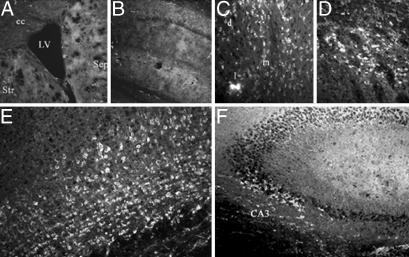
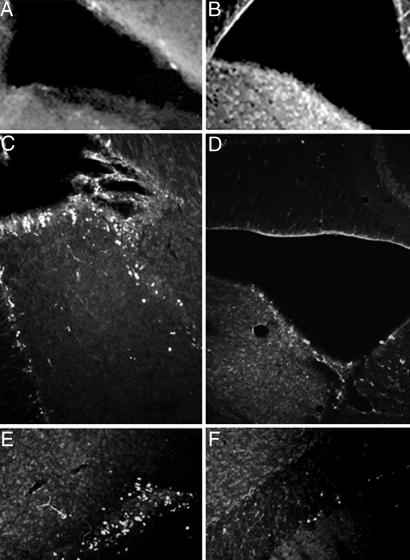
Gene delivery into the brain ventricular space has an advantage of minimizing damage of the brain tissue and could potentially allow expression of the transgenes in several brain structures that surround the ventricles and in cells within the ventricular wall. Seven days after the intraventricular injection of DNA-free ORMOSIL nanoparticles, we found no specific cellular staining within the brain by using anti-EGFP. Fig. 3A shows an area surrounding the left LV, including striatum, septum, and corpus callosum. No EGFP-immunopositive cells were present in any of the areas examined. Fig. 3B illustrates lack of staining in the left hippocampal region of DNA-free ORMOSIL-injected mice. In contrast, the brains of mice injected with ORMOSIL/pEGFP-N2 showed clear cellular EGFP immunofluorescence in the brain structures surrounding the LV. In the septum, medial to the LV, we found EGFP-expressing cells in dorsal lateral intermediate and medial septal nuclei (Fig. 3C). EGFP-expressing cells also were found within the dorsal striatum lateral to the injected ventricle (Fig. 3D). These cells displayed morphology typical of medium spiny neurons, a prevailing neuronal type in striatum. In the adjacent motor cortex, we observed EGFP-immunopositive neuron-shaped cells in several cortical layers (Fig. 3E). These EGFP-expressing cells were densely packed and displayed neuronal morphology with visible neuritic processes. In the hippocampus, EGFP-immunoreactive pyramidal neurons were present in the CA3 area (Fig. 3F).
Fig. 3.
Expression of EGFP in multiple brain areas after injection of ORMOSIL-pEGFP-N2 into the brain LV. Brain sections were immunostained with EGFP antibodies as described in Materials and Methods.(A and B) Control ORMOSIL nanoparticles. (A) The region surrounding the LV. Str, striatum; Sep, septum; cc, corpus callosum. (B) The hippocampal region adjacent to the ventricle. No cellular staining is detected in either region by using anti-EGFP immunocytochemistry. (C–F) ORMOSIL/pEGFP-N2 particles. Injection resulted in EGFP immunostaining of the neuron-shaped cells in dorsal lateral (d), lateral (l), and medial (m) septal nuclei (C); in the adjacent striatal region of the brain (D); cingulate and motor cortex (E); and pyramidal neurons of the CA3 hippocampal region (F).
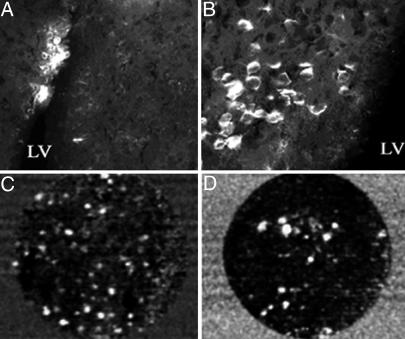
Injection of ORMOSIL/pEGFP-N2 complex into the LV also resulted in EGFP transfection of cells of the SVZ (Fig. 4A). Fig. 4B shows a higher magnification of transfected cells close to the ventricle. Some of these cells also have neuronal-like morphology and could represent maturating neurons. To ascertain that the immunodetected EGFP is expressed in live SVZ cells, we examined whether native EGFP fluorescence could be observed in vivo by using fiber-based confocal fluorescence microscopy (Cell-viZio). Ten days after ORMOSIL/pEGFP-N2 injection into the LV, mice were subjected to a second surgery in which the fiber-optic probe of this instrument was inserted stereotaxically into the ventricle and advanced to the inner ventricular wall. The recorded images indicated a substantial presence of transfected cells in the ventricle wall. The obtained sequences provided information about the spatial distribution of the EGFP-expressing cells in animals without killing them while the probe was lowered into the ventricle. This imaging technology showed that there were more transfected fluorescent cells in the anterior/ventral region (Fig. 4C) than in the posterior region of the LV (Fig. 4D). The fluorescent cells were not visible when the probe was inserted in the center of the ventricle (data not shown). This technique provides a mechanism to monitor in vivo transfection of EGFP or EGFP-tagged fluorescent proteins into localized brain regions to compare gene expression at various times while using a minimal number of animals. This technique also should allow for monitoring, in real-time, division, growth, and/or migration of transfected cells and the effects of genetic manipulations on those processes.
Fig. 4.
Transfection of ORMOSIL-pEGFP-N2 complex into the LV cells of the SVZ. Mice were transfected with ORMOSIL/pEGFP-N2 by injection into the brain LV. (A and B) Seven days postmortem EGFP immunostaining is shown at low magnification (A) and at higher magnification (B) of the positive region to visualize transfected cells. (C and D) In vivo imaging of EGFP fluorescence in cells in the LV. Ten days after transfection, mice were subjected to the second stereotaxic surgery, and a miniature fiber-optic Cell-viZio probe was inserted into the anterior dorsal region (C) or the posterior region (D) of the LV >15 μm from the medial ventricular wall. Dynamic sequences were recorded, and selected frames are shown. The complete dynamic sequences are included in Movie 1, which is published as supporting information on the PNAS web site.
The SVZ of the brain's LVs is richly lined with the NSPCs, which are capable of multiplying, with their progeny migrating through rostral migratory stream to other areas of brain. Given the high efficacy of ORMOSIL-mediated transfection of cells in SVZ, we examined whether this approach may be used to control the biology of these cells. Studies using cultured human and mouse neural progenitor cells have shown that stimulation of the cell surface FGFR1 maintains proliferation of nondifferentiated cells, whereas FGFR1 that accumulates in the nucleus causes the cells to withdraw from the cell cycle and initiate neuronal differentiation (33). In reported studies, immunostaining of freshly isolated mouse brain SVZ cells revealed that all cells expressed FGFR1 and could be stimulated to proliferate by exogenous FGF2 (20, 34). To examine the role of nuclear FGFR1 in the development of brain SVZ cells in situ, we used a nonmembrane/nuclear receptor with the signal peptide replaced by NLS [FGFR1(SP-/NLS)] (35, 36). Mice received intraventricular injection of ORMOSIL/FGFR1(SP-/NLS) nanoparticles or DNA-free ORMOSIL particles, followed 10 days later by BrdUrd injection. Sagittal brain sections were immunostained with mAbs to FGFR1 or BrdUrd. FGFR1 immunostaining was found to be increased in the SVZ of mice transfected with FGFR1(SP-/NLS) (Fig. 5 A and B). Subsequent immunostaining with anti-BrdUrd Ab revealed that a large number of cells in the SVZ (Fig. 5C) and in adjacent rostral migratory stream (Fig. 5E) incorporated BrdUrd in mice transfected with control ORMOSIL nanoparticles. In contrast, in mice transfected with FGFR1(SP-/NLS), only a few cells in each region were stained positive for BrdUrd (Fig. 5 D and F). This effect was not observed with FGFR1(SP-/NLS) with the tyrosine kinase domain (data not shown)
Fig. 5.
Modulation of cell proliferation by using ORMOSIL transfection of nonmembrane/nucleus-targeted FGFR1(SP-/NLS). Control ORMOSIL (A, C, and E) or ORMOSIL/pFGFR1(SP-/NLS) (B, D, and F) was injected into the anterior region of the brain LV. Seven days later, the animals were injected with BrdUrd (i.p.) and were perfused 5 h later. Sagittal brain sections were immunostained for FGFR1 or DNA that had incorporated BrdUrd. (A and B) Immunostaining of SVZ with FGFR1 McAb6. (C and D) BrdUrd immunostaining of cell nuclei in SVZ and adjacent tissue. (E and F) BrdUrd immunostaining of cells in the rostral migratory stream close to SVZ.
Conclusions
ORMOSIL nanoparticles (≈30 nm) are relatively easy to produce on a large scale, and the surfaces of these particles are readily modified during synthesis (10, 25). The addition of cationic groups to the surface of the ORMOSIL enhances binding with negatively charged plasmids for successful carriage inside the cells. This binding provides protection of the sensitive DNA structure from environmental insult during the process involved in in vivo transfer. This process of loading the nanoparticles with DNA for transfection is considerably simpler than the production of transfectable material and its encapsulation in viral particles. The present study demonstrates the ability of this formulation of nanoparticles to effectively traverse a biological barrier and transfect cells in vivo.
The efficiency of ORMOSIL-mediated transfection equaled or exceeded that obtained in previous studies using an herpes simplex viral vector (27). Tissue damage was also observed, caused by associated helper virus during herpes simplex virus 1 vector-mediated intrabrain gene transfer (26, 27). These pathological side effects illustrate problems that are associated with the in vivo gene transfers such as (i) carrier toxicity, (ii) injury due to immunological side effects, and (iii) conversion to a pathogenic form during the transfection process (37–39). In contrast, we observed no such toxic effects in the SN or in brain regions surrounding the LV in mice that received ORMOSIL injections over the time period used in this study. Cells displaying EGFP fluorescence for 10 days after injection indicate that the DNA transfection with ORMOSIL did not cause cellular degeneration. Furthermore, we have injected the same lateral brain ventricle twice 14 days apart (4-week experiment) with ORMOSIL/plasmid DNA and found no evidence of systemic or brain-specific toxicity. This apparent lack of toxicity, together with the exceptionally high efficacy of gene transfection, makes the ORMOSIL nanoparticles a promising experimental and potential therapeutic tool.
In vitro DNA transfection techniques have been used for decades now, and yet no nonviral technique has proven to be as effective as the viral vectors in vivo. Therefore, the transition from in vitro to in vivo systems, as reported here, represents a significant leap forward in the development of experimental techniques to study brain biology and the development of therapeutic approaches to neurological disease. In the present study, we have shown that ORMOSIL nanoparticles can be effectively used to introduce genes into the dopaminergic cells of the SNc. This approach should allow for the modeling of Parkinson's disease, which appears to have a diverse genetic/molecular background, by transfecting with mutant α-synuclein gene, by blocking FGF and glia-derived growth factor signaling with dominant-negative receptor mutants or by knocking down the parkin gene activity by using antisense or small interfering RNA technology (40–43). The ORMOSIL-mediated transfections of the midbrain dopaminergic neurons also would allow testing diverse gene therapeutic strategies for Parkinson's disease as well as for other disorders involving dopaminergic neurons.
The adult mammalian CNS has a limited potential to generate new neurons, making it vulnerable to injury and disease (23). To be able to therapeutically manipulate the endogenous adult NSPCs in the brain SVZ, it is crucial to identify the extracellular and intracellular signals that regulate division and control the fate of these cells (44, 45). In cultured cells and in the developing rat brain, FGFR1 was associated with the peripheral cytoplasm but also with the cell nuclei (46, 47). The cell-surface FGFR mediates the mitogenic effects of extracellular FGFs, whereas the nonmembrane nuclear FGFR1 signals withdrawal from the cell cycle and postmitotic growth (46). FGFR1(SP-/NLS), which does not associate with cell membranes and is expressed specifically in the nucleus, has been shown to stimulate differentiation of human neural progenitor cells in vitro and to directly influence gene activities without affecting the cell survival (28, 36, 48–55). The NSPCs in the adult brain SVZ include relatively quiescent self-renewing pluripotent neural stem cells and developmentally more restricted neural progenitors (35, 56). The single acute BrdUrd injection used in our study is likely to label predominantly the faster-proliferating progenitor cells. The inhibition of BrdUrd incorporation into the cells in SVZ and in rostral migratory stream by transfected FGFR1(SP-/NLS) observed in the present study demonstrates that the nuclear receptor controls the proliferation of the NSPCs in situ. These findings are consistent with our previous in vitro studies that showed that FGFR1(SP-/NLS) induces the neural progenitor cells' withdrawal from the cell cycle and initiates differentiation (46, 57). Thus, ORMOSIL-mediated in vivo transfection of the SVZ cells provides an effective means for elucidating the biology of stem/progenitor cells, allowing for the modification of these developing cells for therapeutic manipulations. ORMOSIL-based interventions could be developed to stimulate neurogenesis and axonal growth, to neutralize potential growth inhibitory molecules, to guide axons to their targets, and to establish new functional synapses. This ability will open perspectives for unraveling mechanisms that control the neural stem cells biology in vivo, providing a promising future direction for effective targeted brain therapy.
In summary, we have developed a synthetic system for the production of an in vivo nonviral transfection vector consisting of amino-terminated ORMOSIL nanoparticles. Intraventricular injection of ORMOSIL/pEGFP-N2 nanoparticles in the mouse brain resulted in the effective transfection and expression of EGFP in neuronal-like cells in periventricular brain regions and the SVZ. In addition, transfection with ORMOSIL/FGFR1(SP-/NLS) nanoparticles resulted in the modulation of the replication cycle of the stem/progenitor cells in the SVZ. These studies provide the groundwork for the use of ORMOSIL nanoparticle formulations for in vivo gene transfer into the CNS and have the potential to provide a safe and efficient mechanism for in vivo gene therapy applications.
Supplementary Material
Acknowledgments
We thank Dr. J. Morys and his research team at the Medical University of Gdansk for sharing their neuroanatomy expertise and Discovery Technology International and Mauna Kea Technologies (Paris) for providing the Cell-viZio fibered confocal in vivo imaging system and the technical support during this study. This work was supported by grants from the Air Force Office of Scientific Research (F496200110358 and FA95500410158, to P.N.P.), the National Science Foundation (DMR-0318211 and NS43621-01, to P.N.P., and IBN-9728923, to M.K.S.), the National Institutes of Health (NS43621, to M.K.S.), the University at Buffalo Interdisciplinary Research and Creative Activities Fund, the John R. Oishei Foundation, the American Parkinson Disease Association, and the Center for Bioinformatics and Life Sciences.
Author contributions: D.J.B., P.N.P., and M.K.S. designed research; D.J.B., I.K., E.K.S., P.D., I.R., and N.K. performed research; D.J.B., I.K., E.K.S., E.J.B., P.N.P., and M.K.S. analyzed data; and D.J.B., E.J.B., and M.K.S. wrote the paper.
Abbreviations: ORMOSIL, organically modified silica; SVZ, subventricular zone; NSPC, neural stem/progenitor cell; FGFR1, FGF receptor type 1; NLS, nuclear localization signal; NGS, normal goat serum; SN, substantia nigra; SNc, SN par compacta; TH, tyrosine hydroxylase; LV, lateral ventricle; SP, signal peptide.
References
- 1.Prasad, P. N. (2003) Introduction to Biophotonics (Wiley, New York).
- 2.Prasad, P. N. (2004) Nanophotonics (Wiley, New York).
- 3.Luo, D. & Saltzman, W. M. (2000) Nat. Biotechnol. 18, 33-37. [DOI] [PubMed] [Google Scholar]
- 4.Davis, S. S. (1997) Trends Biotechnol. 15, 217-224. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, W. F. (1998) Nature 392, 25-30. [DOI] [PubMed] [Google Scholar]
- 6.Luo, D. & Saltzman, W. M. (2000) Nat. Biotechnol. 18, 893-895. [DOI] [PubMed] [Google Scholar]
- 7.Kneuer, C., Sameti, M., Haltner, E. G., Schiestel, T., Schirra, H., Schmidt, H. & Lehr, C. M. (2000) Int. J. Pharm. 196, 257-261. [DOI] [PubMed] [Google Scholar]
- 8.Kneuer, C., Sameti, M., Bakowsky, U., Schiestel, T., Schirra, H., Schmidt, H. & Lehr, C. M. (2000) Bioconjugate Chem. 11, 926-932. [DOI] [PubMed] [Google Scholar]
- 9.He, X. X., Wang, K. M., Tan, W. H., Liu, B., Lin, X., He, C. M., Li, D., Huang, S. S. & Li, J. (2003) J. Am. Chem. Soc. 125, 7168-7169. [DOI] [PubMed] [Google Scholar]
- 10.Roy, I., Ohulchanskyy, T. Y., Mistretta, R. A., Bharali, D. J., Kaur, N., Pudavar, H. & Prasad, P. N. Proc. Natl. Acad. Sci. USA 102, 279-284. [DOI] [PMC free article] [PubMed]
- 11.Das, S., Jain, T. K. & Maitra, A. (2002) J. Colloid Interface Sci. 252, 82-88. [DOI] [PubMed] [Google Scholar]
- 12.Jain, T. K., Roy, I., De, T. K. & Maitra, A. (1998) J. Am. Chem. Soc. 120, 11092-11095. [Google Scholar]
- 13.Toyoda, K., Chu, Y. & Heistad, D. D. (2003) Br. J. Pharmacol. 139, 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Check, E. (2003) Nature 422, 6927. [DOI] [PubMed] [Google Scholar]
- 15.Marwick, C. (2003) Br. Med. J. 326, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn, H. G., Winkler, J., Kempermann, G., Thal, L. J. & Gage, F. H. (1997) J. Neurosci. 17, 5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lois, C. & Alvarez-Buylla, A. (1993) Proc. Natl. Acad. Sci. USA 90, 2074-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman, J. (1995) Blood 85, 1413-1415. [PubMed] [Google Scholar]
- 19.Hauke, J., Helm, M., Lampl, L. & Bock, K. H. (1995) Br. J. Anaesth. 74, 128-128. [Google Scholar]
- 20.Craig, C. G., Tropepe, V., Morshead, C. M., Reynolds, B. A., Weiss, S. & van der Kooy, D. (1996) J. Neurosci. 16, 2649-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raballo, R., Rhee, J., Lyn-Cook, R., Leckman, J. F., Schwartz, M. L. & Vaccarino, F. M. (2000) J. Neurosci. 20, 5012-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horbinski, C., Stachowiak, E. K., Chandrasekaran, V., Miuzukoshi, E., Higgins, D. & Stachowiak, M. K. (2002) J. Neurochem. 80, 54-63. [DOI] [PubMed] [Google Scholar]
- 23.Stachowiak, E. K., Fang, X., Myers, J., Dunham, S. & Stachowiak, M. K. (2003) J. Neurochem. 84, 1296-1312. [DOI] [PubMed] [Google Scholar]
- 24.Reilly, J. F. & Maher, P. A. (2001) J. Cell Biol. 152, 1307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy, I., Ohulchanskyy, T. Y., Pudavar, H. E., Bergey, E. J., Oseroff, A. R., Morgan, J., Dougherty, T. J. & Prasad, P. N. (2003) J. Am. Chem. Soc. 125, 7860-7865. [DOI] [PubMed] [Google Scholar]
- 26.Tabbaa, S., Goulah, W. M., Tran, R. K., Lis, A., Korody, R. Stachowski, B., Horowitz, J. M., Torres, G., Stachowiak, E. K., Bloom, D. C. & Stachowiak, M. D. (2000) Folia Morphol. (Warszawa) 59, 221-232. [PubMed] [Google Scholar]
- 27.Corso, T. D., Toress, G., Roy, I., Gambino, A. S., Nayda, J., Buckley, T., Stachowiak, E. K., Bergey, E. J., Pudavar, H., Dutta, P., et al. (2005) Mol. Brain Res., in press. [DOI] [PubMed]
- 28.Peng, H., Moffett, J., Myers, J., Fang, X. H., Stachowiak, E. K., Maher, P., Kratz, E., Hines, J., Fluharty, S. J., Mizukoshi, E., Bloom, D. C. & Stachowiak, M. K. (2001) Mol. Biol. Cell 12, 449-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanneken, A. (2001) FEBS Lett. 489, 176-181. [DOI] [PubMed] [Google Scholar]
- 30.Wong, A. H. C. & Van Tol, H. H. M. (2003) Neurosci. Biobehav. Rev. 27, 269-306. [DOI] [PubMed] [Google Scholar]
- 31.Fisher, L. J. & Gage, F. H. (1995) Mol. Med. Today 1, 181-187. [DOI] [PubMed] [Google Scholar]
- 32.Fisher, L. J. & Gage, F. H. (1995) Nat. Med. 1, 201-203. [DOI] [PubMed] [Google Scholar]
- 33.Frielingsdorf, H., Schwarz, K., Brundin, P. & Mohapel, P. (2004) Proc. Natl. Acad. Sci. USA 101, 10177-10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gritti, A., Frolichsthal-Schoeller, P., Galli, R., Parati, E. A., Cova, L., Pagano, S. F., Bjornson, C. R. & Vescovi, A. L. (1999) J. Neurosci. 19, 3287-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maslov, A. Y., Barone, T. A., Plunkett, R. J. & Pruitt, S. C. (2004) J. Neurosci. 24, 1726-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno, H., Gunn, M., Dell, K., Tseng, A. & Williams, L. (1992) J. Biol. Chem. 267, 1470-1476. [PubMed] [Google Scholar]
- 37.Yang, K., Clifton, G. L. & Hayes, R. L. (1997) J. Neurotrauma 14, 281-297. [DOI] [PubMed] [Google Scholar]
- 38.Godbey, W. T., Wu, K. K. & Mikos, A. G. (1999) J. Controlled Release 60, 149-160. [DOI] [PubMed] [Google Scholar]
- 39.Geller, A. I., Yu, L., Wang, Y. M. & Fraefel, C. (1997) Exp. Neurol. 144, 98-102. [DOI] [PubMed] [Google Scholar]
- 40.Masliah, E., Rockenstein, E., Veinbergs, I., Sagara, Y., Mallory, M., Hashimoto, M. & Mucke, L. (2001) Proc. Natl. Acad. Sci. USA 98, 12245-12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tooyama, I., McGeer, E. G., Kawamata, T., Kimura, H. & McGeer, P. L. (1994) Brain Res. 656, 165-168. [DOI] [PubMed] [Google Scholar]
- 42.McBride, J. L. & Kordower, J. H. (2002) Prog. Brain Res. 138, 421-432. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg, M. S., Fleming, S. M., Palacino, J. J., Cepeda, C., Lam, H. A., Bhatnagar, A., Meloni, E. G., Wu, N. P., Ackerson, L. C., Klapstein, G. J., et al. (2003) J. Biol. Chem. 278, 43628-43635. [DOI] [PubMed] [Google Scholar]
- 44.Cattaneo, E. & McKay, R. (1991) Trends Neurosci. 14, 338-340. [DOI] [PubMed] [Google Scholar]
- 45.Gage, F. H. (1994) Neurobiol. Aging 15, S191. [DOI] [PubMed] [Google Scholar]
- 46.Stachowiak, M. K., Fang, X. H., Myers, J. M., Dunham, S. M., Berezney, R., Maher, P. A. & Stachowiak, E. K. (2003) J. Cell. Biochem. 90, 662-691. [DOI] [PubMed] [Google Scholar]
- 47.Hu, Y. F., Fang, X. H., Dunham, S. M., Prada, C., Stachowiak, E. K. & Stachowiak, M. K. (2004) J. Biol. Chem. 279, 29325-29335. [DOI] [PubMed] [Google Scholar]
- 48.Morshead, C. M. & van der Kooy, D. (1992) J. Neurosci. 12, 249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wichterle, H., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (1997) Neuron 18, 779-791. [DOI] [PubMed] [Google Scholar]
- 50.Allen, E. (1912) J. Comp. Neurol. 22, 547-568. [Google Scholar]
- 51.Smart, I. (1961) J. Comp. Neurol. 116, 325-338. [Google Scholar]
- 52.Peng, H., Myers, J., Fang, X. H., Stachowiak, E. K., Maher, P. A., Martins, G. G., Popescu, G., Berezney, R. & Stachowiak, M. K. (2002) J. Neurochem. 81, 506-524. [DOI] [PubMed] [Google Scholar]
- 53.Fang, X., Stachowiak, E. K., Dunham-Ems, S. M., Klejbor, I. & Stachowiak, M. K. (2005) J. Biol. Chem., in press. [DOI] [PubMed]
- 54.Shin, J. H., London, J., Le Pecheur, M., Hoger, H., Pollak, D. & Lubec, G. (2004) Free Radical Biol. Med. 37, 643-653. [DOI] [PubMed] [Google Scholar]
- 55.Shin, D. M., Korada, S., Raballo, R., Shashikant, C. S., Simeone, A., Taylor, J. R. & Vaccarino, F. (2004) J. Neurosci. 24, 2247-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morshead, C. M., Reynolds, B. A., Craig, C. G., McBurney, M. W., Staines, W. A., Morassutti, D., Weiss, S. & van der Kooy, D. (1994) Neuron 13, 1071-1082. [DOI] [PubMed] [Google Scholar]
- 57.Stachowiak, M. K. (2003) J. Neurochem. 85, 13-13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.