Abstract
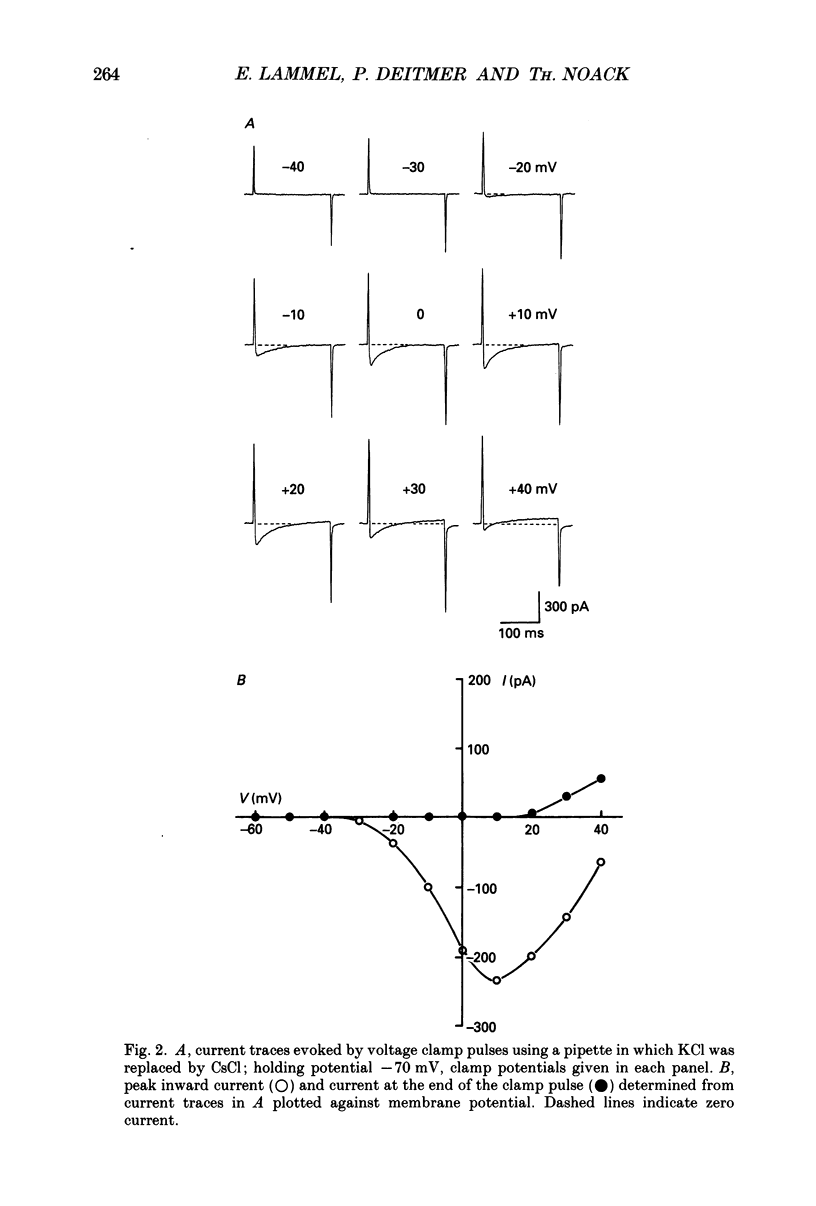
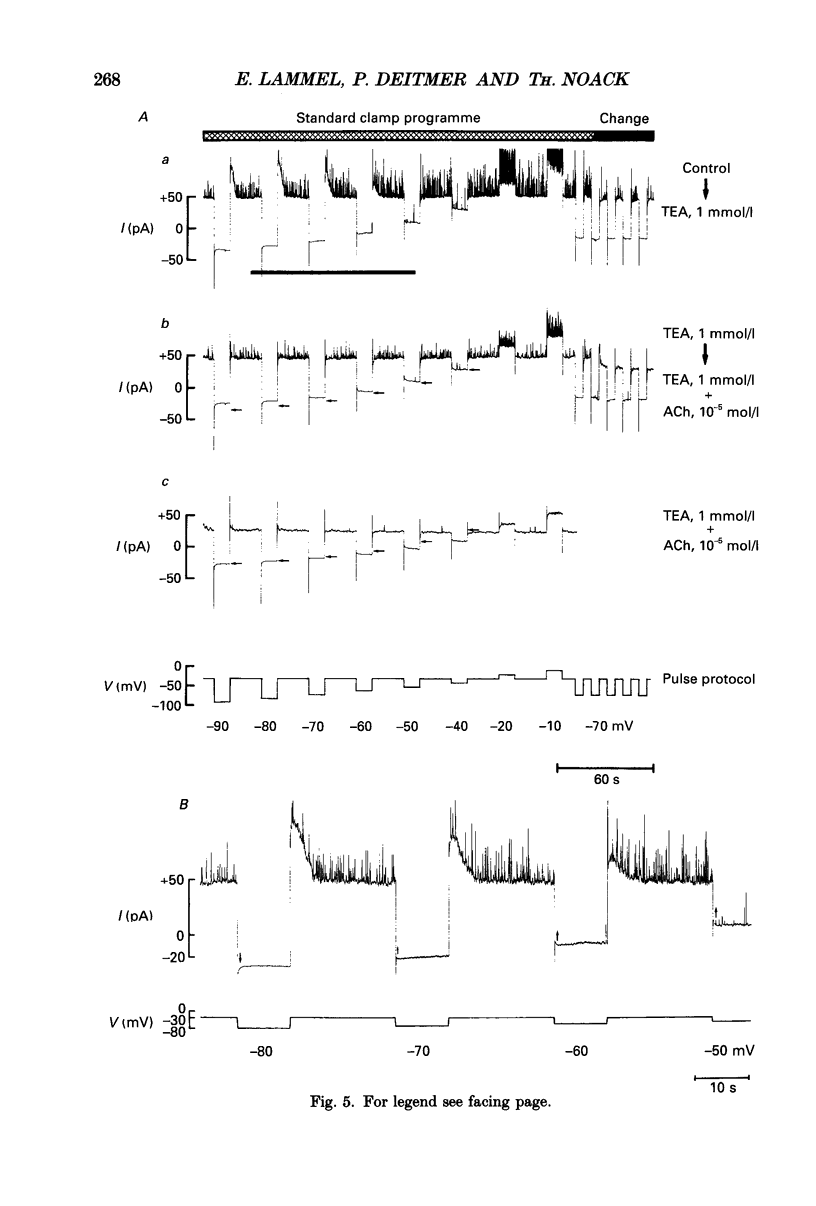
1. Single smooth muscle cells from the fundus region of the guinea-pig stomach, which showed contractile responses to acetylcholine (ACh) at concentrations greater than or equal to 10(-7) mol/l, were obtained by enzymatic digestion using highly purified collagenase and papain. They were studied by recording membrane currents under voltage clamp with the patch pipette technique in the whole-cell configuration at 25-28 degrees C. 2. By applying voltage jumps from negative holding levels (-70 to -60 mV) to more positive levels, we identified two major activating currents: an initial inward Ca2+ current (ICa) was followed, and partly overlapped, by an outward K+ current (IK). 3. Cholinergic effects on membrane currents were investigated in the range of negative membrane potentials by determining current-voltage relations in the absence of ACh and during its continuous presence in the bathing fluid. 4. ACh induced a decrease in the steady-state conductance which was reversibly blocked by atropine. At physiological external K+ concentration [( K+]o = 6 mmol/l), the reversal potential (Erev) of the current suppressed by ACh (3 x 10(-6) mol/l) was about 20 mV more positive than the calculated K+ equilibrium potential (EK). 5. When [K+]o was increased, Erev was shifted positively; but at each [K+]o, Erev was more positive than EK. 6. Like ACh (10(-6) mol/l), tetraethylammonium (TEA, 1 mmol/l) also suppressed a current with a reversal potential that was, at physiological [K+]o, 20 mV more positive than EK. ACh (10(-5) mol/l) applied in the presence of 1 mmol/l TEA suppressed a pure K+ current (Erev = EK), which was also suppressed by 10 mmol/l TEA. 7. When K+ in the pipette and in the bathing solution was completely replaced by Na+, both ACh (10(-5) mol/l) and TEA (1 mmol/l) caused a reduction of the membrane conductance that appeared to be identical. TEA added to the bathing solution in the presence of ACh did not produce a significant additional conductance decrease. These results did not depend on whether Cl- was present as a charge carrier or not. 8. It is concluded that in fundus muscle of the guinea-pig stomach a major mechanism underlying muscarinic activation is a decrease of a K+ conductance. In addition the results indicate a suppression of a small Na+ conductance which is made up by a population of channels that are also blocked by TEA.
Full text
PDF





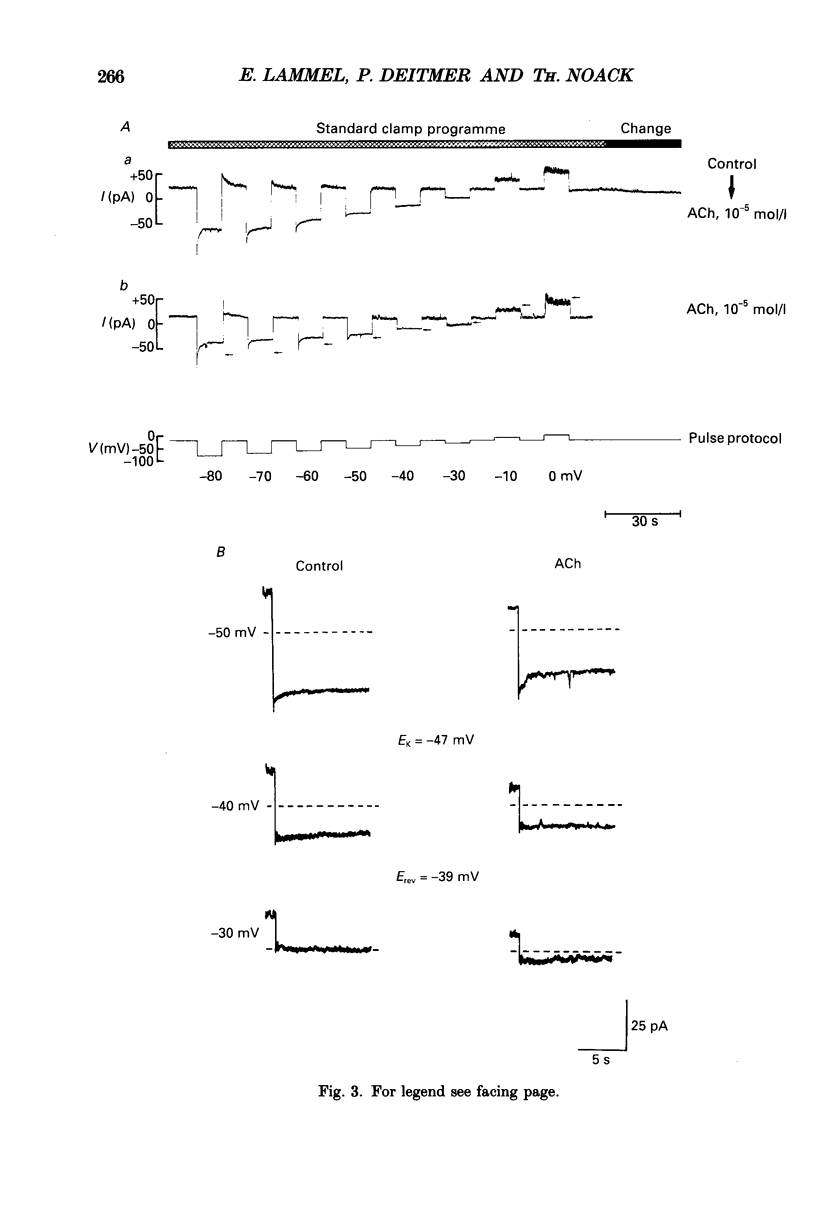
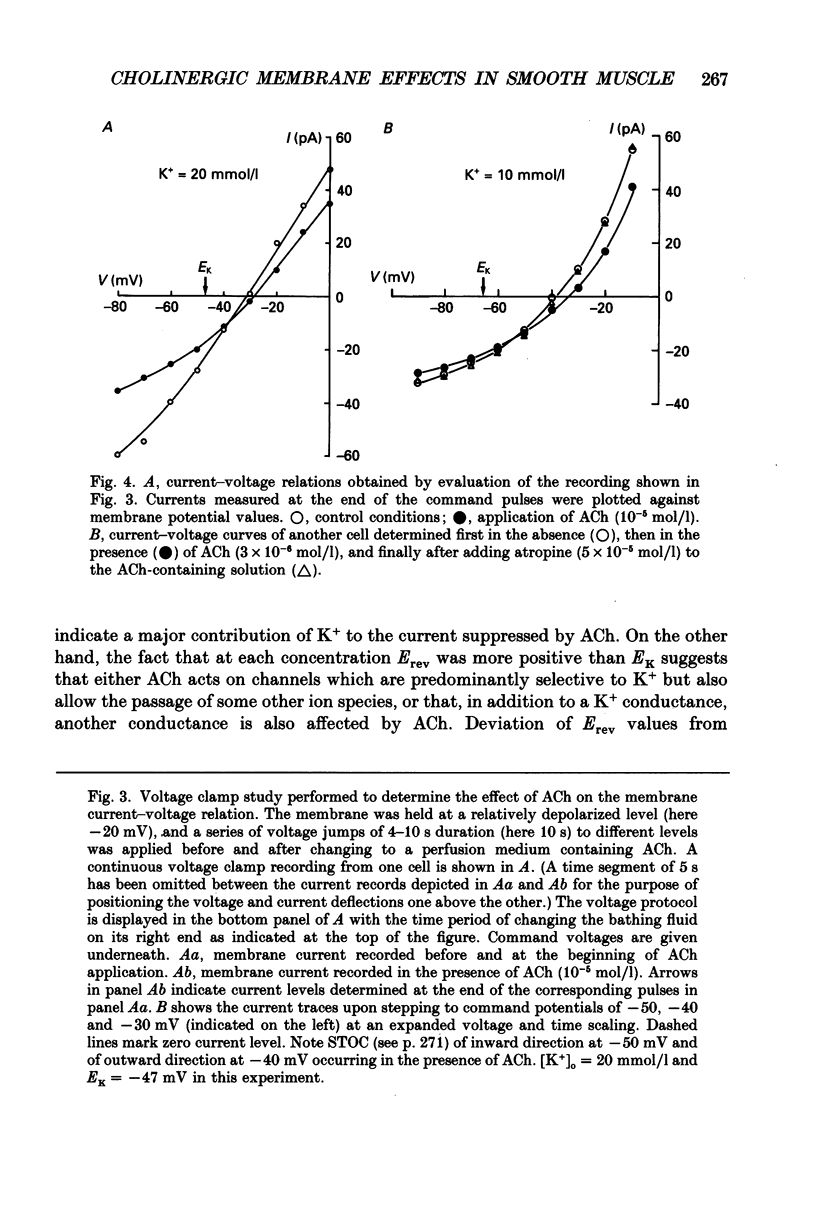










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Kitamura K. Evidence that ionic channels associated with the muscarinic receptor of smooth muscle may admit calcium. Br J Pharmacol. 1983 Feb;78(2):405–416. doi: 10.1111/j.1476-5381.1983.tb09405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986 Dec 4;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- Golenhofen K., von Loh D., Milenov K. Elektrophysiologische Untersuchungen zur Spontanaktivität isolierter Muskelpräparate aus verschiedenen Abschnitten des Meerschweinchen-Magens. Pflugers Arch. 1970;315(4):336–356. doi: 10.1007/BF00593460. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Actions of noradrenaline and acetylcholine on sympathetic ganglion cells. J Physiol. 1970 Jun;208(2):353–372. doi: 10.1113/jphysiol.1970.sp009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel E. A theoretical study on the sucrose gap technique as applied to multicellular muscle preparations. I. Saline-sucrose interdiffusion. Biophys J. 1981 Dec;36(3):533–553. doi: 10.1016/S0006-3495(81)84751-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel E. A theoretical study on the sucrose gap technique as applied to multicellular muscle preparations. II. Methodical errors in the determination of outward currents. Biophys J. 1981 Dec;36(3):555–573. doi: 10.1016/S0006-3495(81)84752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama I., Yoshida C., Kobayashi M., Oyamada H., Momose K. Preparation of single smooth muscle cells from guinea pig taenia coli by combinations of purified collagenase and papain. J Pharmacol Methods. 1987 Sep;18(2):151–161. doi: 10.1016/0160-5402(87)90008-8. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Singer J. J., Walsh J. V., Jr Cholinergic agonists suppress a potassium current in freshly dissociated smooth muscle cells of the toad. J Physiol. 1985 Oct;367:503–529. doi: 10.1113/jphysiol.1985.sp015837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Presser F., Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988 Apr 8;240(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Kuba K. Muscarinic regulation of two ionic currents in the bullfrog sympathetic neurone. Pflugers Arch. 1988 Apr;411(4):361–370. doi: 10.1007/BF00587714. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Minota S., Kuba K. Regulation of two ion channels by a common muscarinic receptor-transduction system in a vertebrate neuron. Neurosci Lett. 1987 Oct 16;81(1-2):139–145. doi: 10.1016/0304-3940(87)90354-5. [DOI] [PubMed] [Google Scholar]


