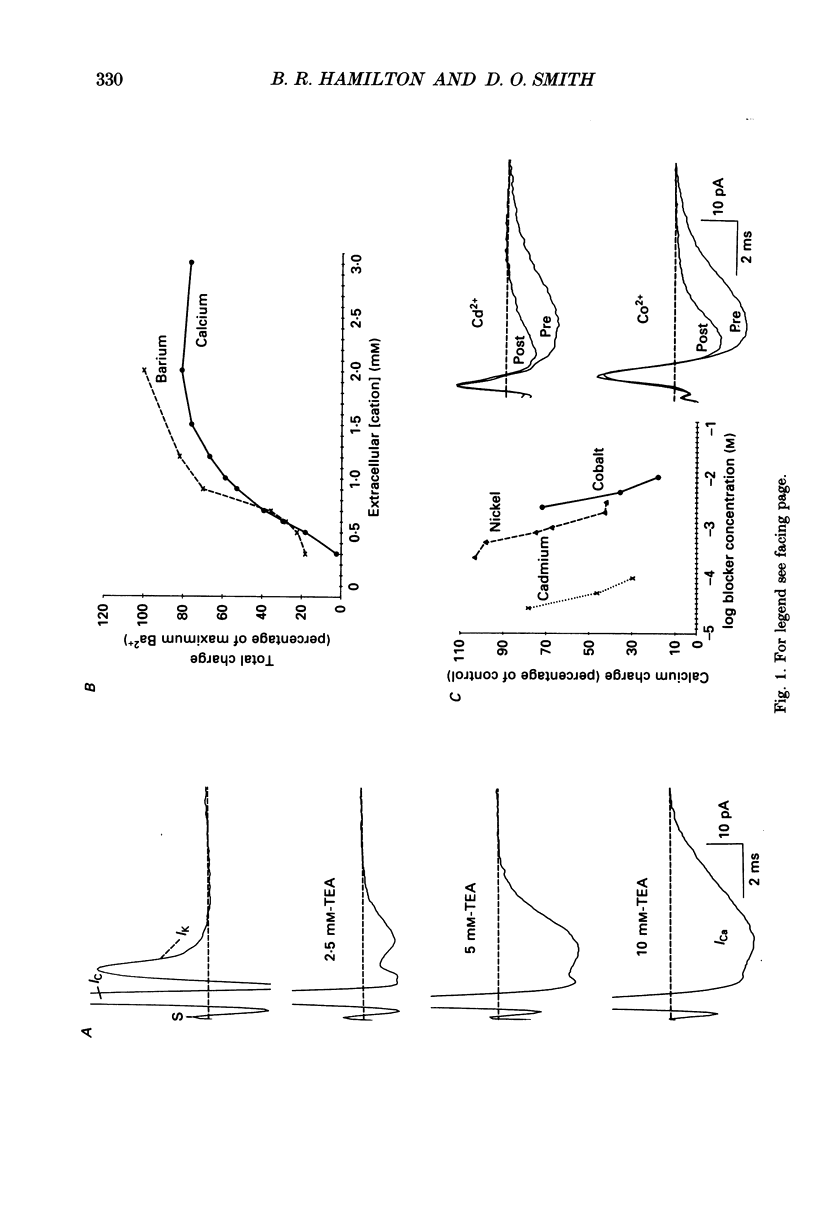
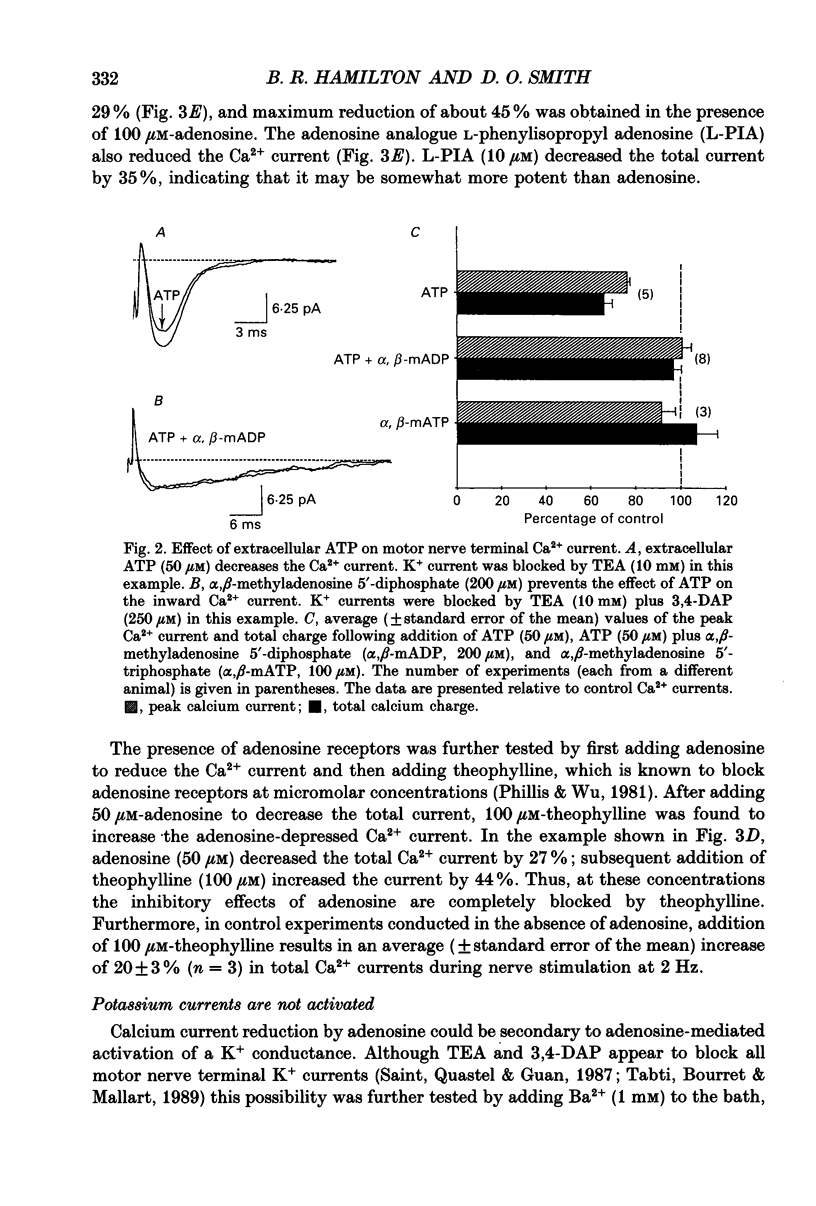
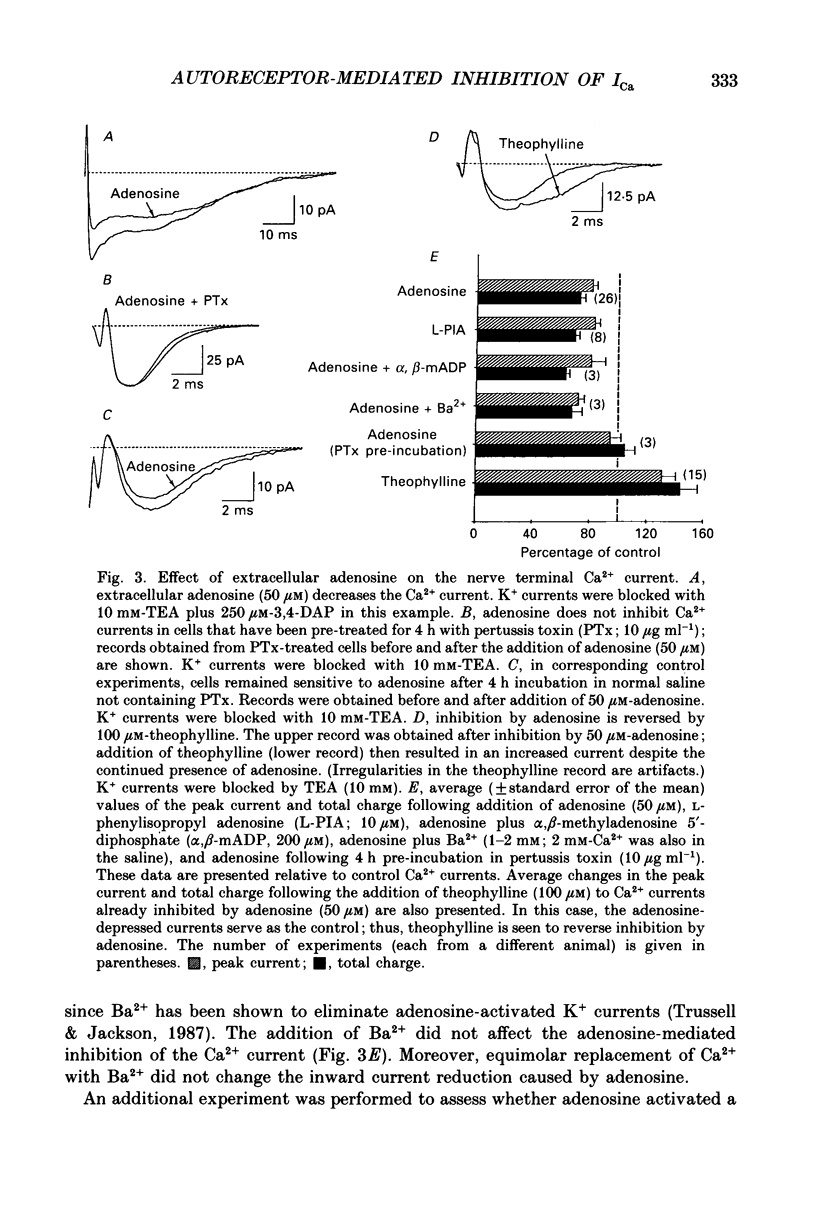
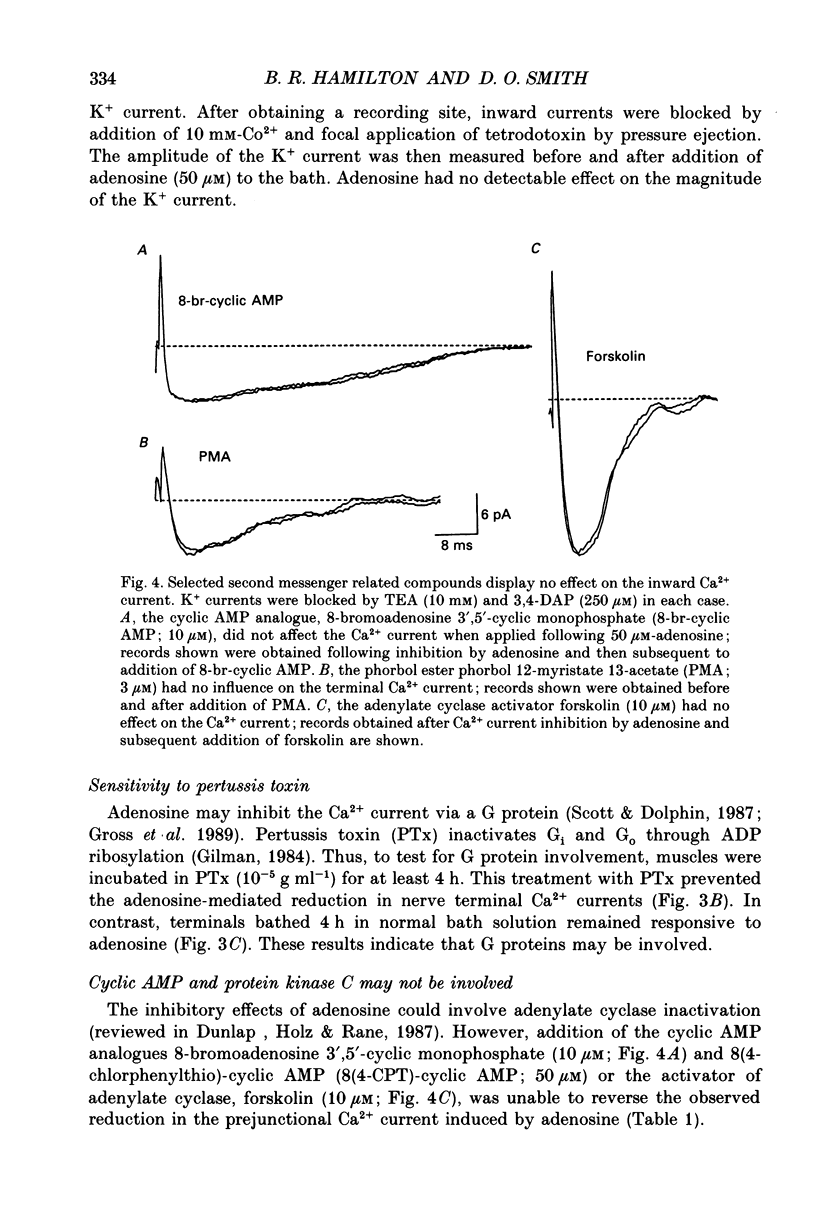
Abstract
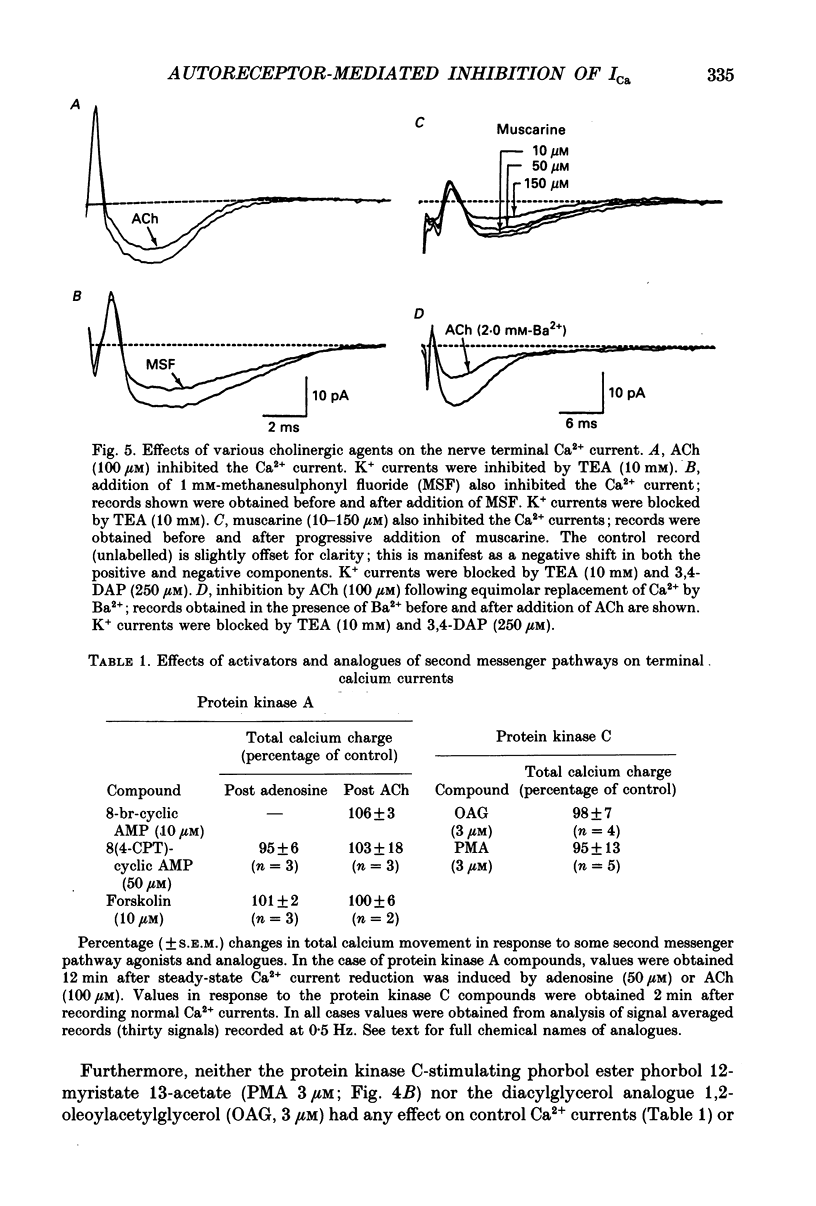
1. After blocking K+ currents with 10 mM-tetraethylammonium (TEA) or TEA plus 250 microM-3,4-diaminopyridine (3,4-DAP). motor nerve terminal Ca2+ currents were recorded using focal extracellular electrodes. Two transmitters released from the terminal. ATP and acetylcholine (ACh), were then applied, and the effects on the nerve terminal Ca2+ current were measured. 2. ATP (50 microM) reduced the Ca2+ current by 34%, but this action is prevented when hydrolysis to adenosine is blocked by alpha,beta-methyladenosine 5'-diphosphate (200 microM). Thus, inhibition by ATP presumably occurs subsequent to ATP hydrolysis to adenosine. 3. Adenosine (50 microM) inhibited the terminal Ca2+ current by 29%. This was mimicked by the adenosine analogue L-phenylisopropyl adenosine (L-PIA) and blocked by theophylline (100 microM), which antagonizes adenosine receptors at micromolar concentrations. 4. ACh (100 microM) or the anticholinesterase methane sulphonyl fluoride (MSF; 1 mM) also depressed the terminal Ca2+ current. This response was mimicked by muscarine (100 microM) and antagonized by atropine (100 microM) or pirenzipine (4 microM), which is generally specific for M1 receptors. 5. Addition of Ba2+, which blocks adenosine-mediated K+ currents, had no effect on the inhibitory effects of either adenosine or ACh; similarly, neither adenosine nor ACh in the bath affected K+ current records obtained after blocking all inward currents with 10 mM-Co2+ and focal application of tetrodotoxin. 6. Incubation of the muscle for 4 h in pertussis toxin (10(-5) g ml-1) eliminated both adenosine- and ACh-induced inhibition of the terminal Ca2+ current. This result indicates the possible involvement of a G protein in the transduction of the feedback pathway. 7. Neither cyclic AMP analogues, the adenylate cyclase activator forskolin (10 microM), the phorbol ester phorbol 12-myristate 13-acetate (PMA; 3 microM) nor the diacylglycerol analogue 1,2-oleoylacetylglycerol (OAG; 3 microM) had any effect on adenosine- or ACh-induced depression of the terminal Ca2+ current. Therefore, pathways involving these particular second messengers are most probably not involved. 8. The effects of adenosine and ACh are non-additive. 9. These results indicate that ATP and ACh, which are released during exocytosis, may inhibit their own release through attenuation of the terminal Ca2+ current via autoreceptors coupled to a G protein.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. Preparation and properties of 5'-nucleotidase from smooth muscle of small intestine. J Biol Chem. 1970 Dec 10;245(23):6274–6280. [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M., Murphy K., Leslie R. A., Robertson H. A. Localization of adenosine A1-receptors to the terminals of the perforant path. Brain Res. 1988 Oct 18;462(2):252–257. doi: 10.1016/0006-8993(88)90553-7. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L., Ryan-Jastrow T. 2-Chloroadenosine reduces the N calcium current of cultured mouse sensory neurones in a pertussis toxin-sensitive manner. J Physiol. 1989 Apr;411:585–595. doi: 10.1113/jphysiol.1989.sp017592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. Muscarine affects calcium-currents in rat hippocampal pyramidal cells in vitro. Neurosci Lett. 1987 May 19;76(3):301–306. doi: 10.1016/0304-3940(87)90419-8. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Salpeter M. M. Absence of [125I] alpha-bungarotoxin binding to motor nerve terminals of frog, lizard and mouse muscle. J Neurosci. 1983 Feb;3(2):326–331. doi: 10.1523/JNEUROSCI.03-02-00326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Lindgren C. A., Moore J. W. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol. 1989 Jul;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Alpha-Bungarotoxin enhances transmitter "released" at the neuromuscular junction. Nature. 1978 Apr 13;272(5654):641–643. doi: 10.1038/272641a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Dreyer F. Two different presynaptic calcium currents in mouse motor nerve terminals. Pflugers Arch. 1986 Feb;406(2):190–197. doi: 10.1007/BF00586682. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Reddington M., Lee K. S., Schubert P. An A1-adenosine receptor, characterized by [3H] cyclohexyladenosine binding, mediates the depression of evoked potentials in a rat hippocampal slice preparation. Neurosci Lett. 1982 Mar 5;28(3):275–279. doi: 10.1016/0304-3940(82)90070-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol. 1987 Mar;384:571–585. doi: 10.1113/jphysiol.1987.sp016470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint D. A., Quastel D. M., Guan Y. Y. Multiple potassium conductances at the mammalian motor nerve terminal. Pflugers Arch. 1987 Nov;410(4-5):408–412. doi: 10.1007/BF00586518. [DOI] [PubMed] [Google Scholar]
- Schubert P., Heinemann U., Kolb R. Differential effect of adenosine on pre- and postsynaptic calcium fluxes. Brain Res. 1986 Jun 25;376(2):382–386. doi: 10.1016/0006-8993(86)90204-0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. Evidence for specific adenosine receptors at cholinergic nerve endings. Br J Pharmacol. 1980;71(1):191–194. doi: 10.1111/j.1476-5381.1980.tb10925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Acetylcholine synthesis and release in the extensor digitorum longus muscle of mature and aged rats. J Neurochem. 1990 Apr;54(4):1433–1439. doi: 10.1111/j.1471-4159.1990.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Smith D. O. Sources of adenosine released during neuromuscular transmission in the rat. J Physiol. 1991 Jan;432:343–354. doi: 10.1113/jphysiol.1991.sp018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabti N., Bourret C., Mallart A. Three potassium currents in mouse motor nerve terminals. Pflugers Arch. 1989 Feb;413(4):395–400. doi: 10.1007/BF00584489. [DOI] [PubMed] [Google Scholar]
- Toselli M., Lux H. D. GTP-binding proteins mediate acetylcholine inhibition of voltage dependent calcium channels in hippocampal neurons. Pflugers Arch. 1989 Jan;413(3):319–321. doi: 10.1007/BF00583548. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W., Thierry D. L. Adenosine receptor agonists inhibit K+-evoked Ca2+ uptake by rat brain cortical synaptosomes. J Neurochem. 1982 Sep;39(3):700–708. doi: 10.1111/j.1471-4159.1982.tb07949.x. [DOI] [PubMed] [Google Scholar]
- Yatani A., Hamm H., Codina J., Mazzoni M. R., Birnbaumer L., Brown A. M. A monoclonal antibody to the alpha subunit of Gk blocks muscarinic activation of atrial K+ channels. Science. 1988 Aug 12;241(4867):828–831. doi: 10.1126/science.2457252. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L., Inestrosa N. C., Hall Z. W. Acetylcholinesterase is functional in embryonic rat muscle before its accumulation at the sites of nerve-muscle contact. Dev Biol. 1984 Jun;103(2):369–377. doi: 10.1016/0012-1606(84)90325-7. [DOI] [PubMed] [Google Scholar]


