Abstract
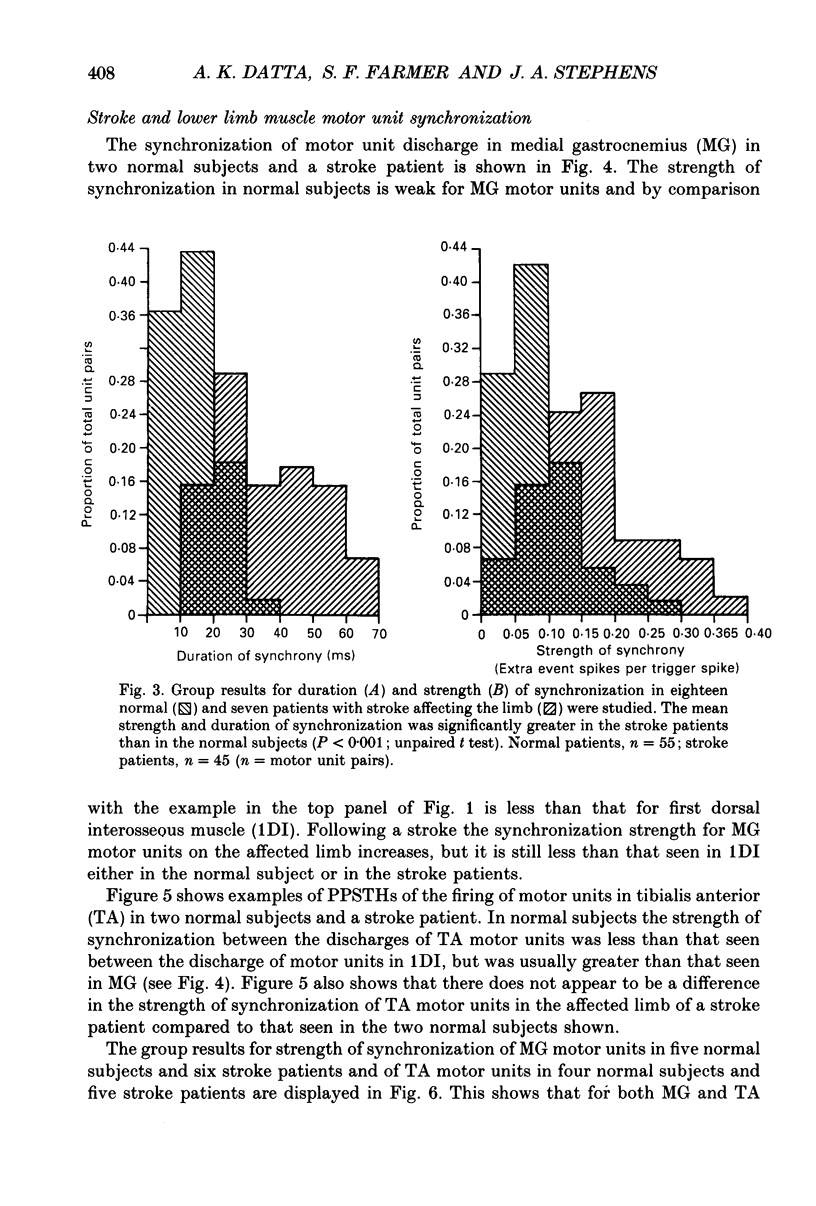
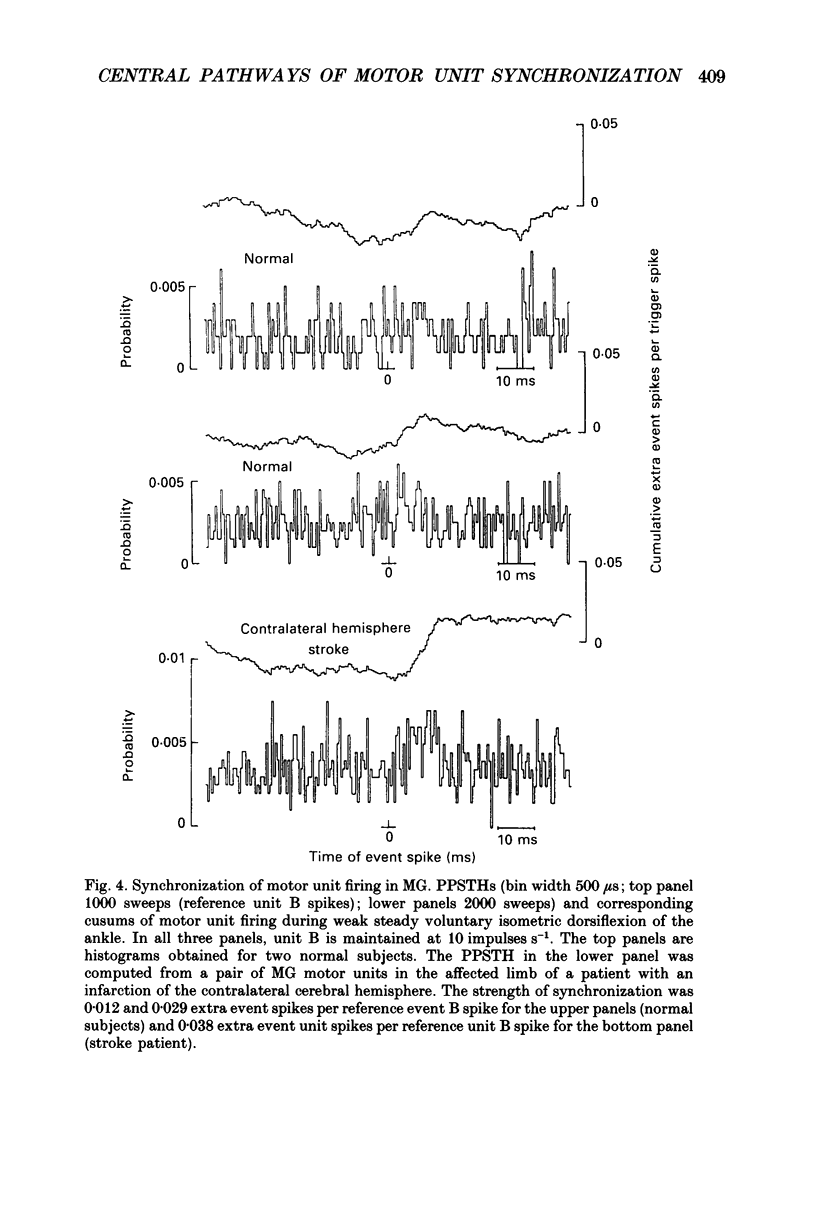
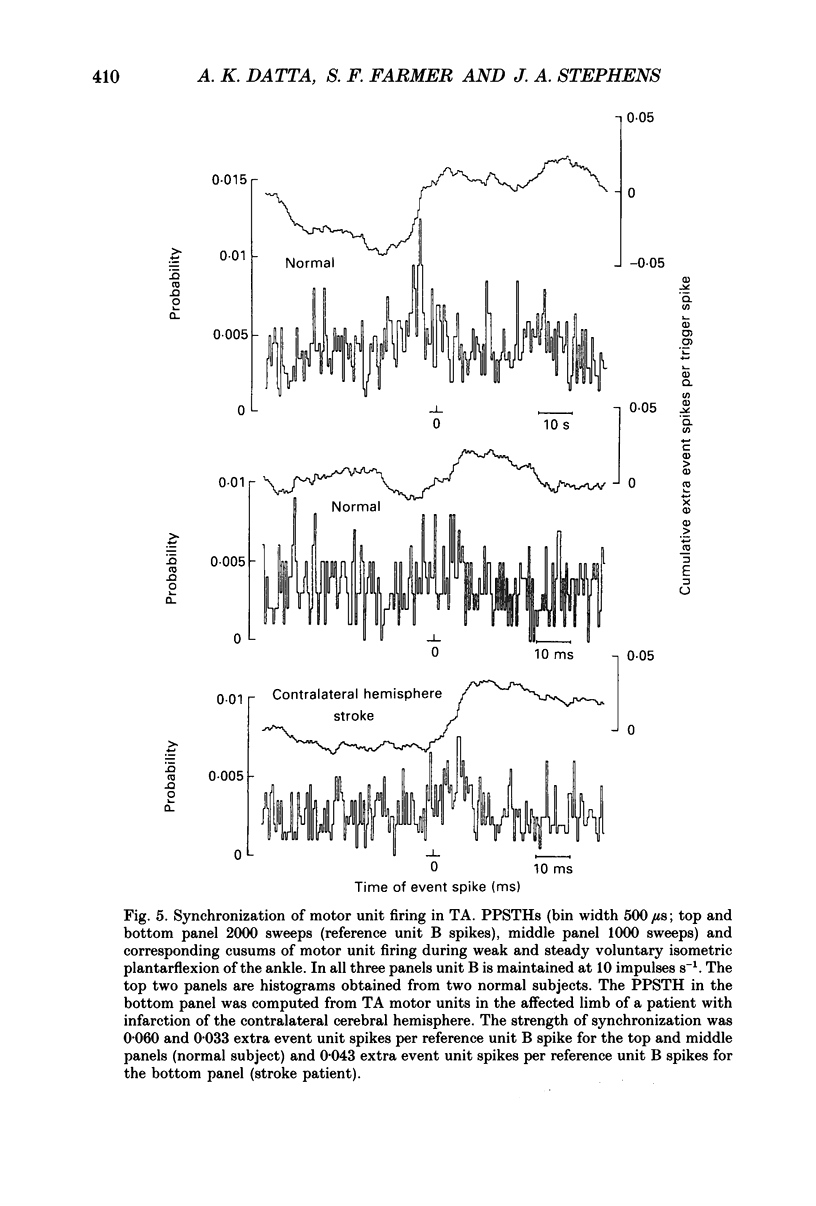
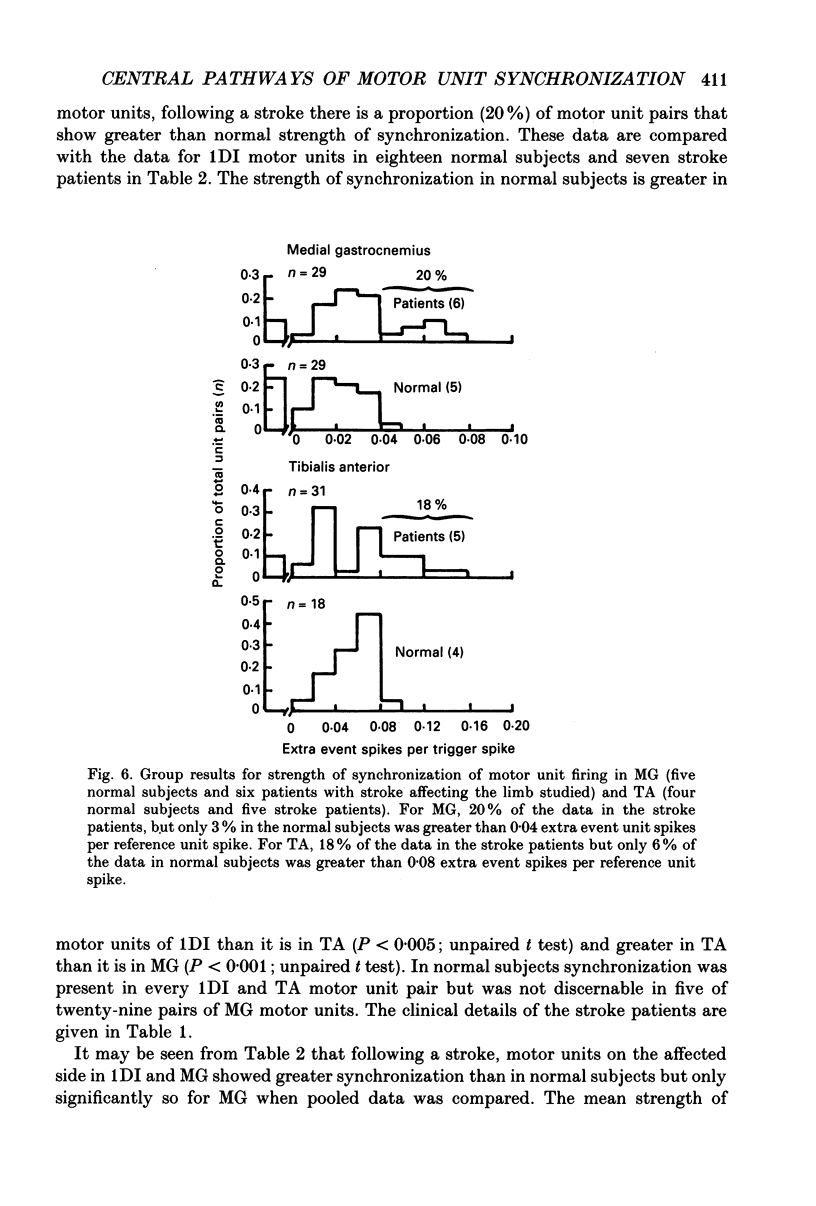
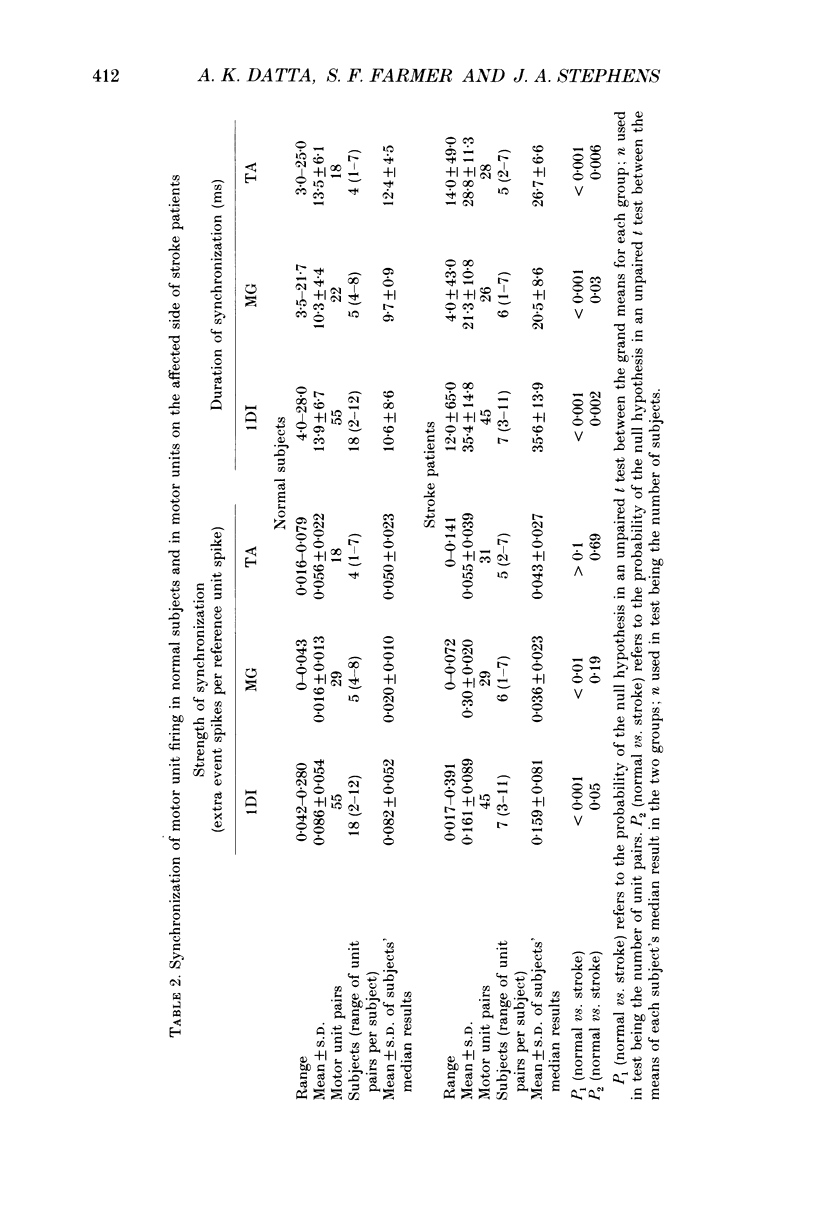
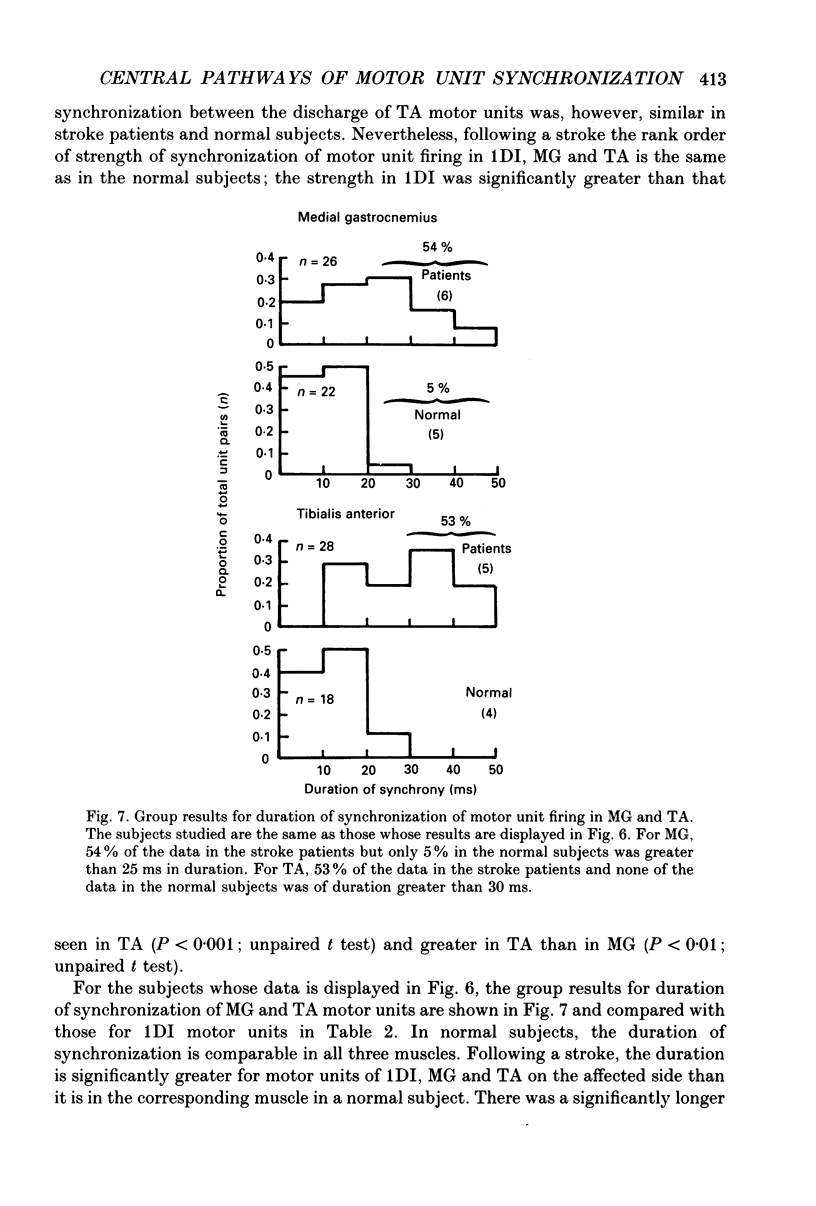
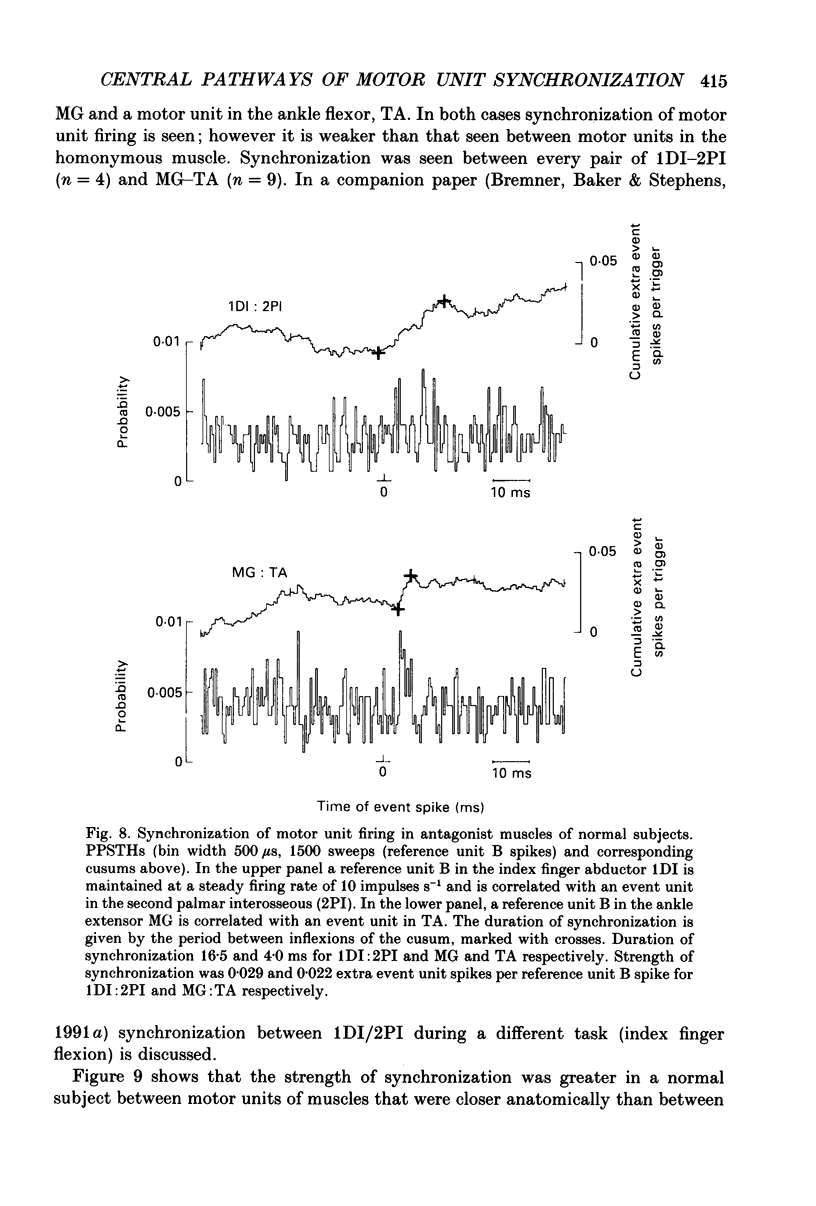
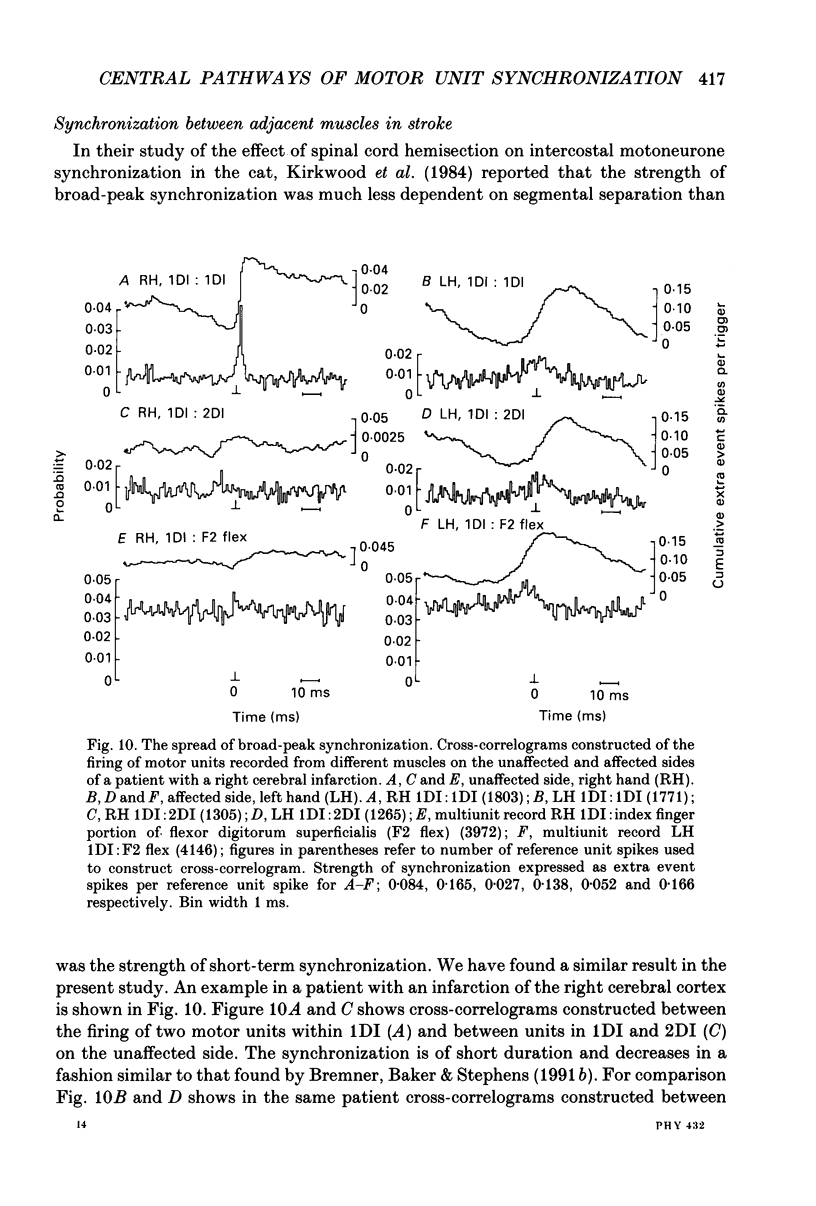
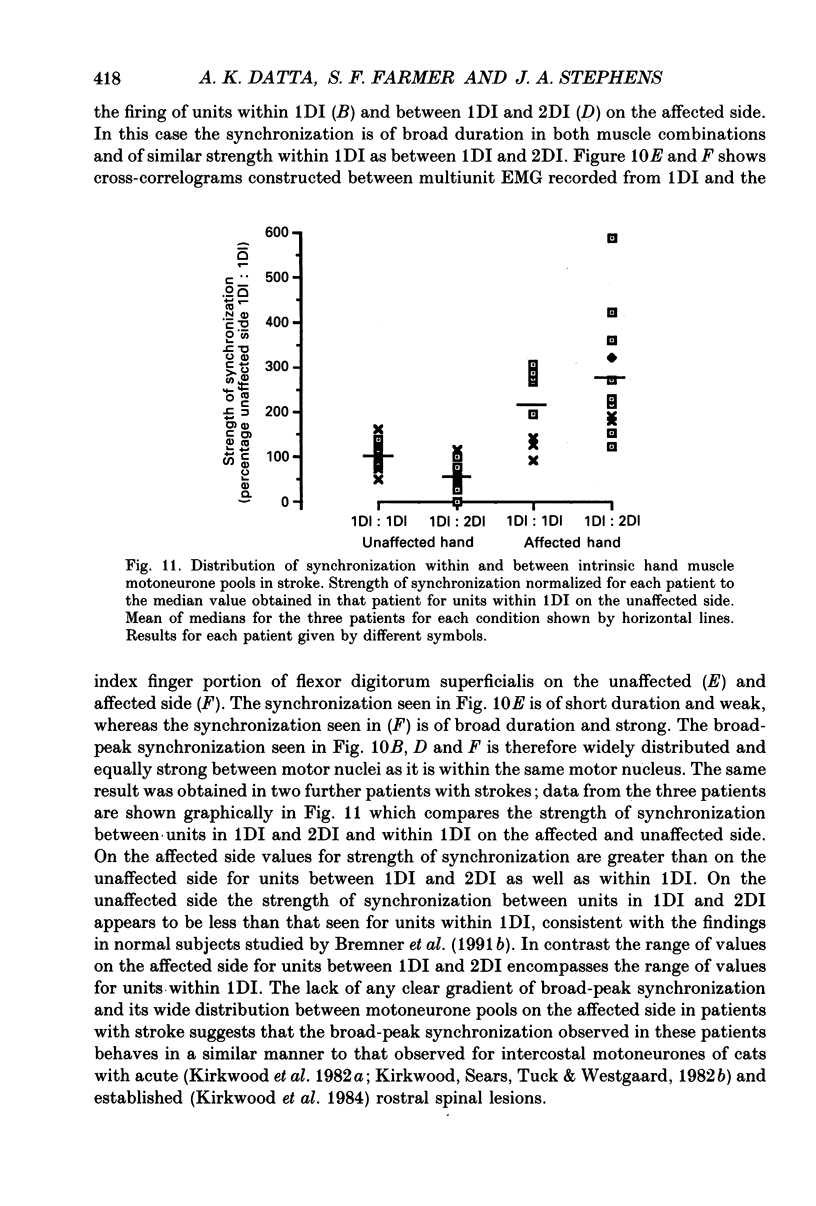
1. Motor unit firing has been studied during weak voluntary isometric contractions with pairs of needle electrodes in normal human subjects. 2. Pre- and post-stimulus time histograms of the firing time of firing of one event unit before and after the time of firing of another reference (stimulus) unit showed a clear central peak, indicative of synchronization. 3. Synchronization was seen in all the muscles studied. The mean strength of synchronization, expressed as the number of concomitant discharges of the two units as a proportion of the number of stimulus unit discharges, was 0.095 extra event unit spikes/reference unit spike (range 0.042-0.28) for first dorsal interosseous muscle, 0.016 extra event unit spikes per reference unit spike (range 0-0.043) for medial gastrocnemius and 0.056 extra event unit spikes per reference unit spike range 0.016-0.079) for tibialis anterior. 4. The mean duration of synchronization was 11.3 ms (range 5.0-21.0 ms) for first dorsal interosseous, 10.3 ms (range 3.5-21.7 ms) for medial gastrocnemious and 13.5 ms (range 3.0-25.0) for tibialis anterior. 5. Seven patients with radiographically and clinically identified central strokes were studied while they made weak voluntary isometric contractions. The duration of synchronization was significantly prolonged compared to that found in normal subjects. In these stroke patients the mean duration of synchronization on the affected side was longer than that seen in the normal subjects, and in first dorsal interosseous muscle was 35.4 ms (range 12.0-65.0 ms), in medial gastrocnemius was 21.3 ms (range 4.0-43.0 ms) and in tibialis anterior was 28.8 ms (range 14.0-49.0 ms). 6. The mean strength of synchronization of motor unit discharge was found to be greater in the stroke patients than that seen in the normal subjects for first dorsal interosseous muscle (0.161 extra event unit spikes per reference unit spike, range 0.017-0.391) and for medial gastrocnemius (0.030 extra event unit spikes per reference unit spike) but only significantly so when pooled data was compared. There was no difference in the strength of motor unit synchronization in tibialis anterior between stroke patients and normal subjects. 7. Broad duration synchronization among first dorsal interosseous motor units was also found in a patient with a rostral cervical spine lesion (total duration range 43-46 ms; n = 2), but not in a patient with a caudal (thoracic) spinal lesion.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L., Datta A. K., Guz A. Synchronization of motor unit firing during different respiratory and postural tasks in human sternocleidomastoid muscle. J Physiol. 1989 Jun;413:213–231. doi: 10.1113/jphysiol.1989.sp017650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H., Zarzecki P., Jankowska E., Hongo T., Marcus S. Projection of individual pyramidal tract neurons to lumbar motor nuclei of the monkey. Exp Brain Res. 1979 Jan 2;34(1):73–89. doi: 10.1007/BF00238342. [DOI] [PubMed] [Google Scholar]
- Bremner F. D., Baker J. R., Stephens J. A. Correlation between the discharges of motor units recorded from the same and from different finger muscles in man. J Physiol. 1991 Jan;432:355–380. doi: 10.1113/jphysiol.1991.sp018389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner F. D., Baker J. R., Stephens J. A. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. J Physiol. 1991 Jan;432:381–399. doi: 10.1113/jphysiol.1991.sp018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi S. Ultrastructure of dorsal root boutons on lumbosacral motoneurons of the adult cat, as revealed by dorsal root section. Acta Physiol Scand Suppl. 1969;332:85–115. [PubMed] [Google Scholar]
- Datta A. K., Stephens J. A. Synchronization of motor unit activity during voluntary contraction in man. J Physiol. 1990 Mar;422:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler R., Wolf W., Schubert M., Struppler A. Discharge pattern of single motor units in basal ganglia disorders. Neurology. 1986 Aug;36(8):1061–1066. doi: 10.1212/wnl.36.8.1061. [DOI] [PubMed] [Google Scholar]
- Dietz V., Bischofberger E., Wita C., Freund H. J. Correlation between the dischanges of two simultaneously recorded motor units and physiological tremor. Electroencephalogr Clin Neurophysiol. 1976 Jan;40(1):97–105. doi: 10.1016/0013-4694(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. An application of cumulative sum technique (cusums) to neurophysiology [proceedings]. J Physiol. 1977 Feb;265(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Muscle fields of primate corticomotoneuronal cells. J Physiol (Paris) 1978;74(3):239–245. [PubMed] [Google Scholar]
- Harrison P. J., Taylor A. Individual excitatory post-synaptic potentials due to muscle spindle Ia afferents in cat triceps surae motoneurones. J Physiol. 1981 Mar;312:455–470. doi: 10.1113/jphysiol.1981.sp013638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUYPERS H. G., FLEMING W. R., FARINHOLT J. W. Subcorticospinal projections in the rhesus monkey. J Comp Neurol. 1962 Feb;118:107–137. doi: 10.1002/cne.901180109. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D., Westgaard R. H. The spatial distribution of synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:137–155. doi: 10.1113/jphysiol.1982.sp014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol. 1984 May;350:225–251. doi: 10.1113/jphysiol.1984.sp015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCOUCH G. P., AUSTIN G. M., LIU C. N., LIU C. Y. Sprouting as a cause of spasticity. J Neurophysiol. 1958 May;21(3):205–216. doi: 10.1152/jn.1958.21.3.205. [DOI] [PubMed] [Google Scholar]
- Powers R. K., Rymer W. Z. Effects of acute dorsal spinal hemisection on motoneuron discharge in the medial gastrocnemius of the decerebrate cat. J Neurophysiol. 1988 May;59(5):1540–1556. doi: 10.1152/jn.1988.59.5.1540. [DOI] [PubMed] [Google Scholar]
- Pullen A. H., Sears T. A. Modification of "C" synapses following partial central deafferentation of thoracic motoneurones. Brain Res. 1978 Apr 21;145(1):141–146. doi: 10.1016/0006-8993(78)90802-8. [DOI] [PubMed] [Google Scholar]
- Pullen A. H., Sears T. A. Trophism between C-type axon terminals and thoracic motoneurones in the cat. J Physiol. 1983 Apr;337:373–388. doi: 10.1113/jphysiol.1983.sp014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. S. On Nerve-Tracts degenerating secondarily to Lesions of the Cortex Cerebri. J Physiol. 1889 Jul;10(5):429–432. doi: 10.1113/jphysiol.1889.sp000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y., Zarzecki P., Asanuma H. Spinal branching of pyramidal tract neurons in the monkey. Exp Brain Res. 1979 Jan 2;34(1):59–72. doi: 10.1007/BF00238341. [DOI] [PubMed] [Google Scholar]
- Stein R. D., Weaver L. C. Multi- and single-fibre mesenteric and renal sympathetic responses to chemical stimulation of intestinal receptors in cats. J Physiol. 1988 Feb;396:155–172. doi: 10.1113/jphysiol.1988.sp016956. [DOI] [PMC free article] [PubMed] [Google Scholar]