Abstract
1. Calcium currents (ICa) were measured in frog ventricular myocytes using the whole-cell patch clamp technique and a perfused pipette. The effect of internal perfusion with the hydrolysis-resistant GTP analogue, GppNHp (5'guanylylimidodiphosphate), on basal ICa and ICa stimulated with forskolin or isoprenaline was examined to gain insight into the role of G proteins in ICa regulation. 2. Without added guanine nucleotides, isoprenaline stimulated ICa approximately 14-fold with an EC50 of 0.09 microM. Forskolin stimulated ICa approximately 10-fold with an EC50 of 0.30 microM. 3. Internal 30 microM-GppNHp produced an approximately 80% decrease in ICa elevated by 0.3 microM-isoprenaline or 3 microM-forskolin. The inhibition of isoprenaline stimulation was due to a decrease in the maximal stimulation from approximately 14-fold to approximately 14-fold without a significant change in the EC50. In contrast, the reduction in forskolin stimulation was due to a 22-fold increase in the EC50 to 11.4 microM, with little change in maximal stimulation. 4. The inhibition of stimulated ICa by GppNHp is likely to be mediated by a G protein, because the effects of GppNHp are irreversible, and are blocked by excess GTP. ICa is affected similarly by GppNHp and by ACh. This suggests that GppNHp activates the same G protein that is normally activated by ACh, but activation by GppNHp occurs in the absence of agonist occupation of the muscarinic receptor. 5. The increase in the EC50 for forskolin produced by internal GppNHp was reversed by exposure to isoprenaline, which itself did not affect ICa amplitude. On average, exposure to isoprenaline in the presence of GppNHp caused an irreversible 81-fold decrease in the EC50 for forskolin to 0.14 microM. Stimulation of ICa by forskolin after internal GppNHp and exposure to isoprenaline was completely blocked by the protein kinase A inhibitor PKI(5-22). 6. These effects do not involve the phospholipase C system, because they are not mimicked by phorbol esters or internal inositol 1,4,5-trisphosphate (IP3) and are not blocked by bromophenacyl bromide or neomycin. 7. Direct effects of G proteins on ICa were not evident, because internal perfusion with PKI(5-22) completely inhibited isoprenaline- or forskolin-stimulated increases in ICa, and neither ACh nor internal GppNHp (30-500 microM) affected basal ICa or ICa elevated by internally perfused cyclic AMP. 8. These results suggest that the predominant site of action of the inhibitory G protein activated by either GppNHp or ACh is adenylyl cyclase. Furthermore, the internally perfused frog cardiomyocytes may provide a useful approach for probing the detailed interactions of G proteins, forskolin, and adenylyl cyclase in an intact cell.
Full text
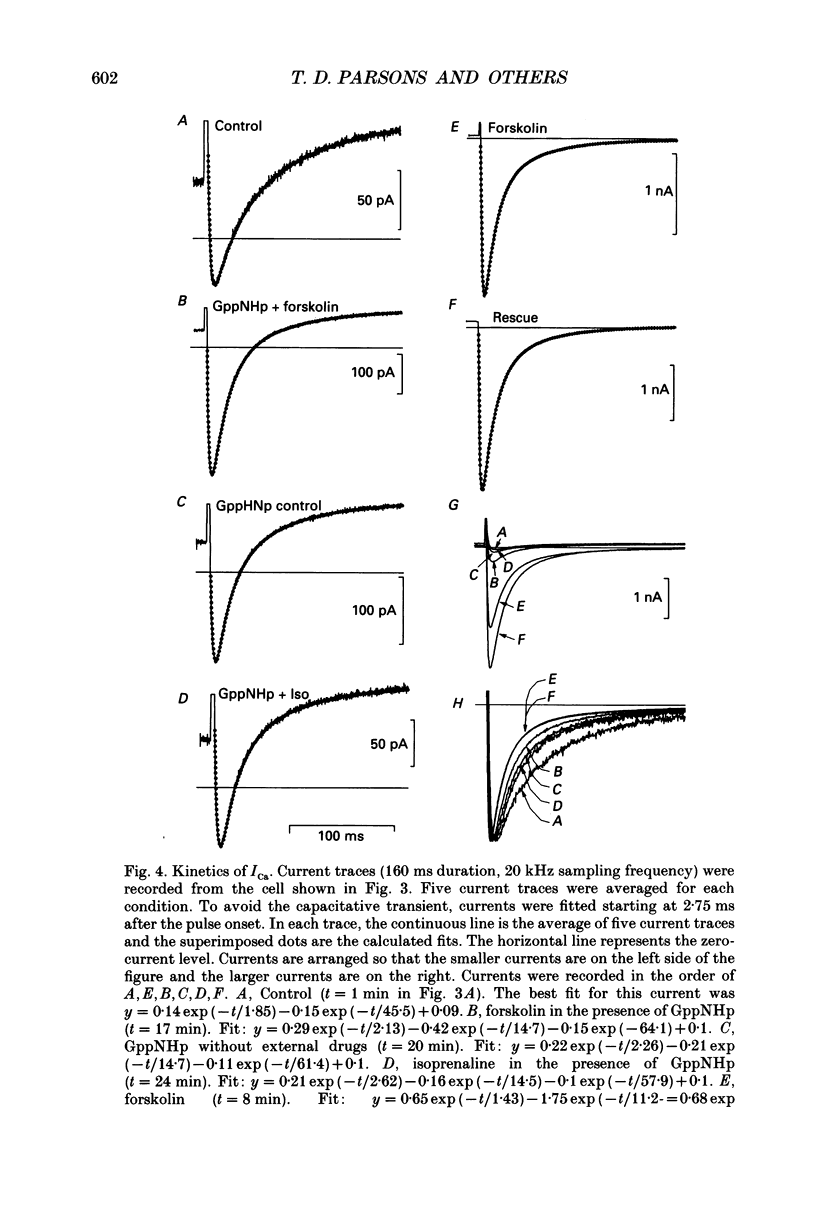
PDF





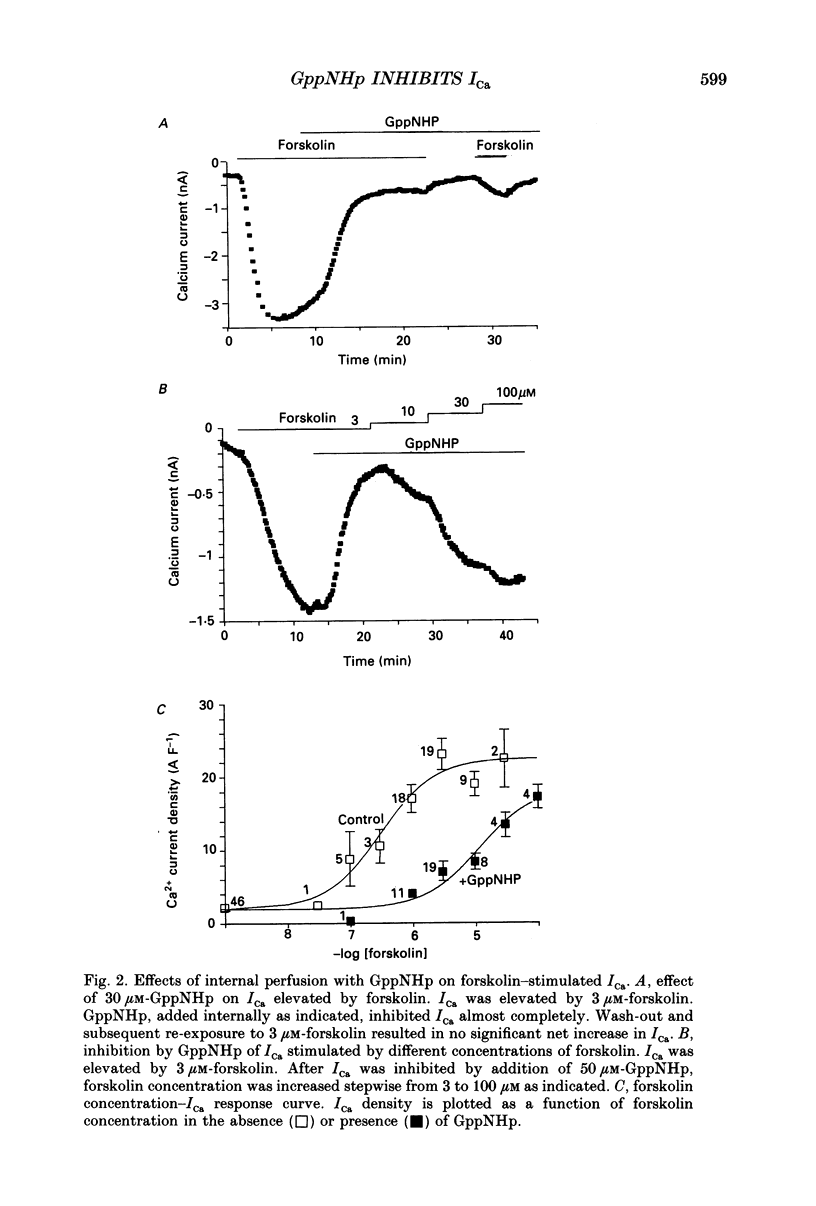


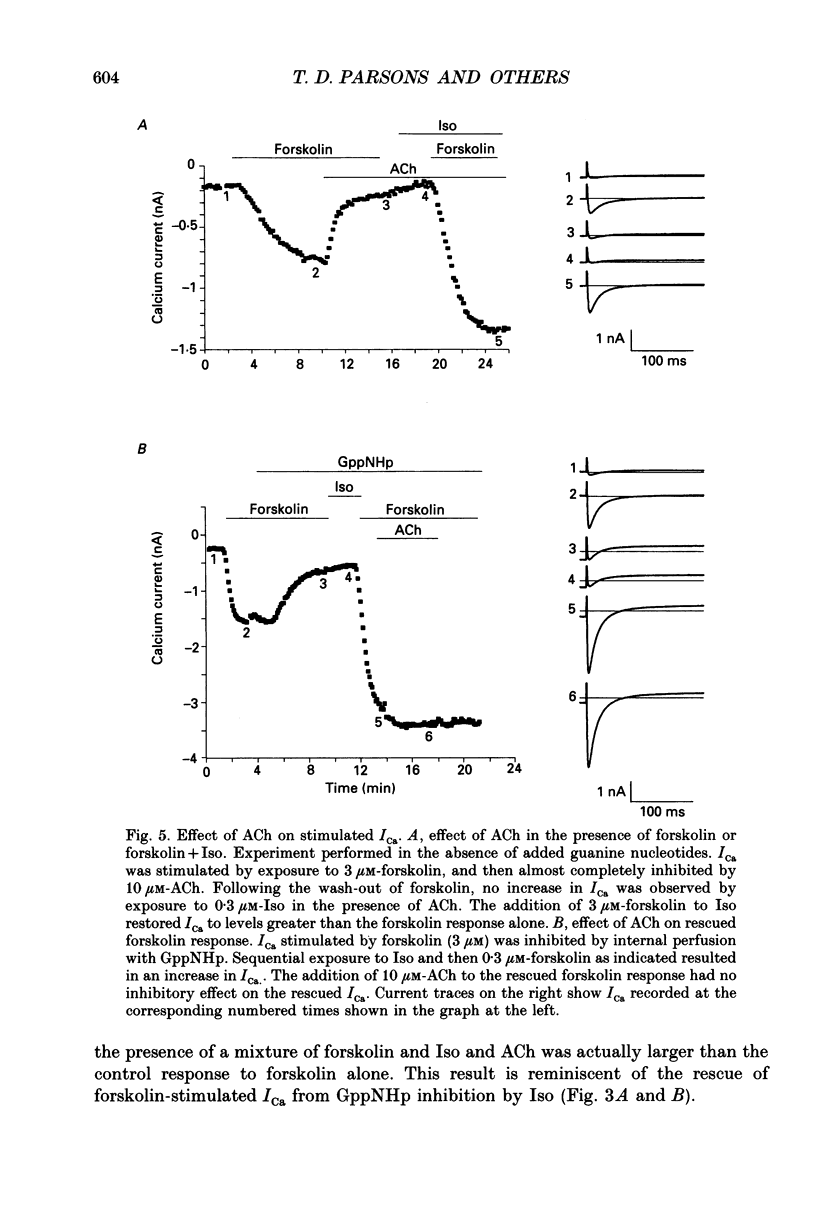
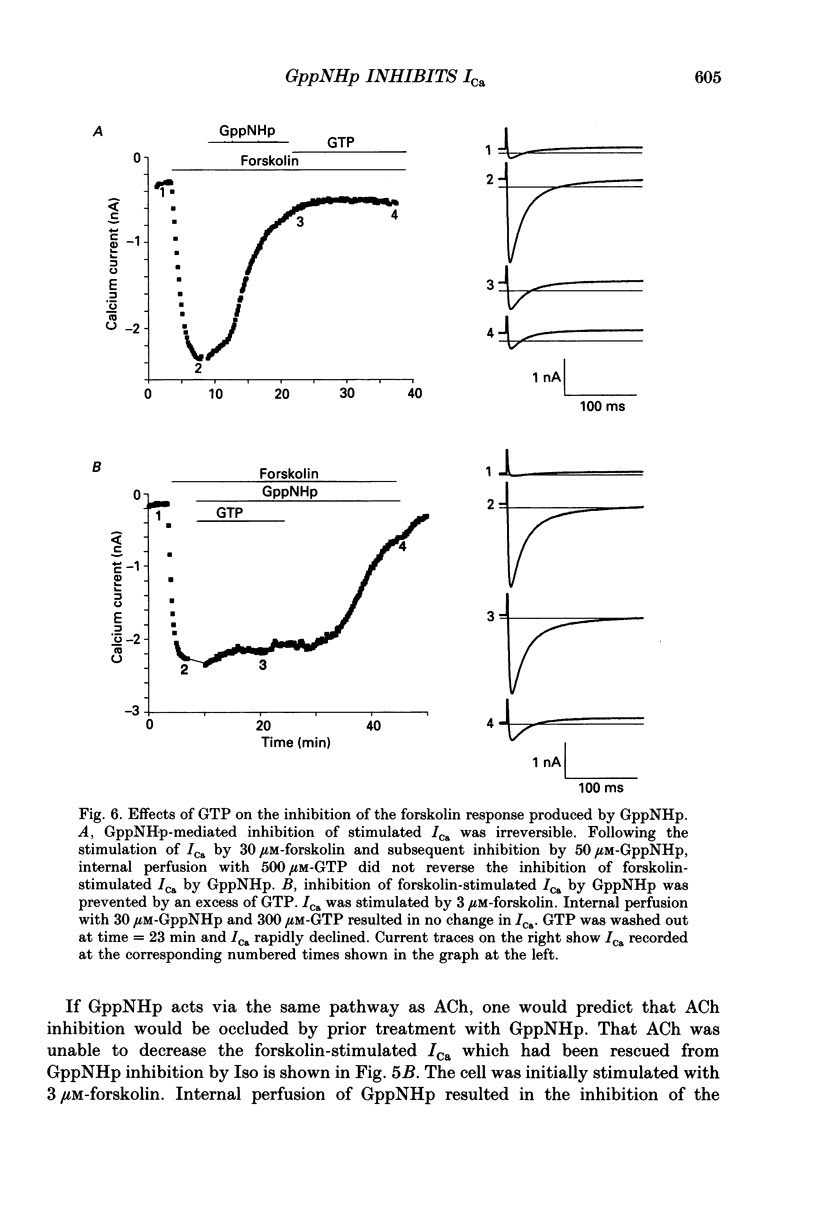

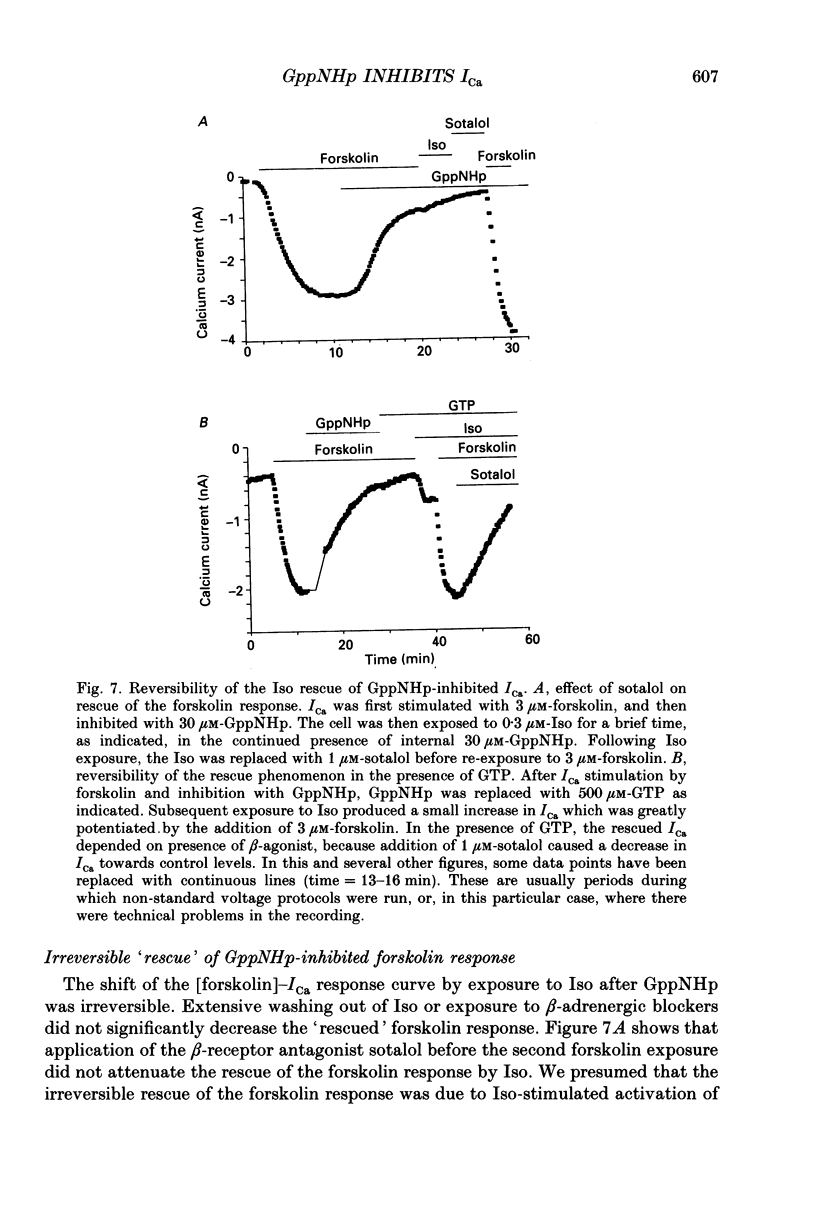













Selected References
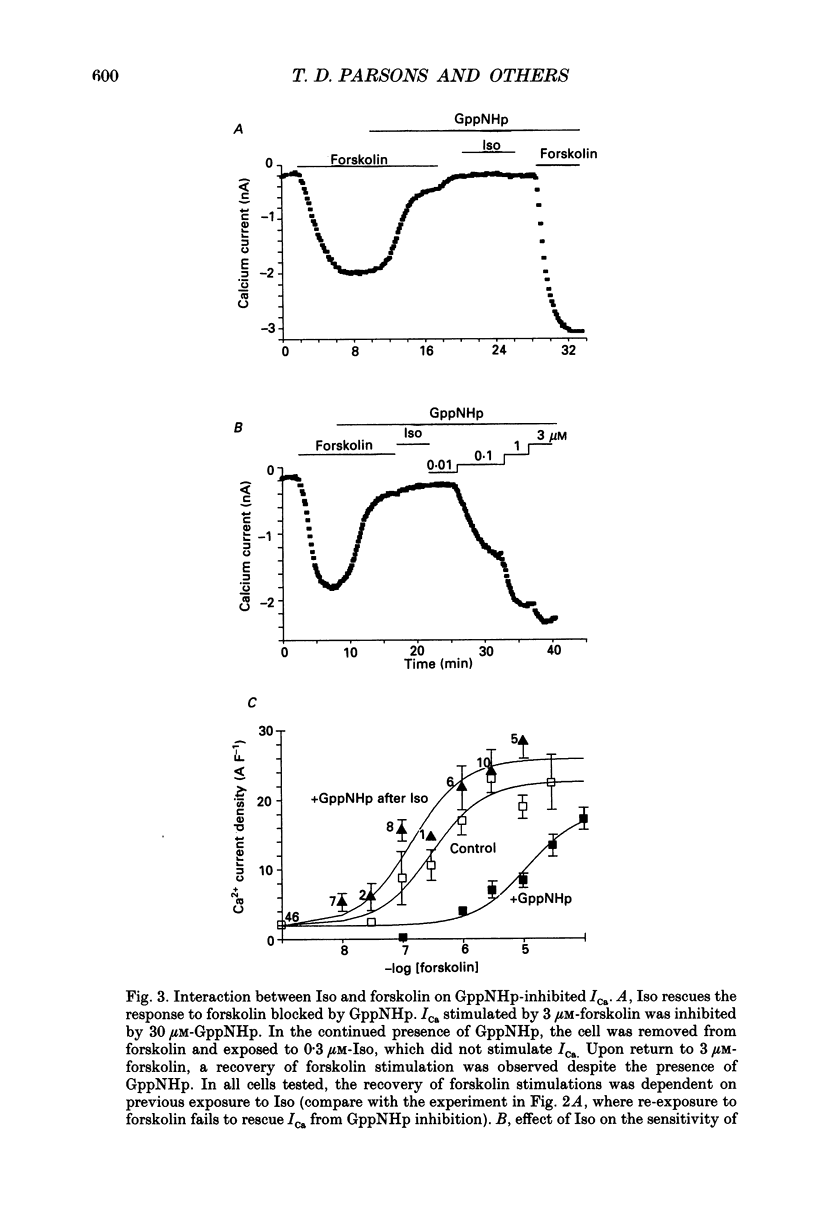
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell G. J., Flower R. J. Inhibition of phospholipase. Br Med Bull. 1983 Jul;39(3):260–264. doi: 10.1093/oxfordjournals.bmb.a071830. [DOI] [PubMed] [Google Scholar]
- Brandt D. R., Ross E. M. Catecholamine-stimulated GTPase cycle. Multiple sites of regulation by beta-adrenergic receptor and Mg2+ studied in reconstituted receptor-Gs vesicles. J Biol Chem. 1986 Feb 5;261(4):1656–1664. [PubMed] [Google Scholar]
- Brandwein H. J., Lewicki J. A., Waldman S. A., Murad F. Effect of GTP analogues on purified soluble guanylate cyclase. J Biol Chem. 1982 Feb 10;257(3):1309–1311. [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Gierschik P., Staniszewski C., Benovic J. L., Codina J., Somers R., Birnbaumer L., Spiegel A. M., Lefkowitz R. J., Caron M. G. Functional differences in the beta gamma complexes of transducin and the inhibitory guanine nucleotide regulatory protein. Biochemistry. 1987 Mar 10;26(5):1485–1491. doi: 10.1021/bi00379a041. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Staniszewski C., Gierschik P., Codina J., Somers R. L., Birnbaumer L., Spiegel A. M., Caron M. G., Lefkowitz R. J. Mechanism of guanine nucleotide regulatory protein-mediated inhibition of adenylate cyclase. Studies with isolated subunits of transducin in a reconstituted system. J Biol Chem. 1986 Jul 15;261(20):9514–9520. [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Birnbaumer L., Sekura R. D. Effects of guanine nucleotides and Mg on human erythrocyte Ni and Ns, the regulatory components of adenylyl cyclase. J Biol Chem. 1984 Sep 25;259(18):11408–11418. [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Shrier A. Interactive effects of isoprenaline, forskolin and acetylcholine on Ca2+ current in frog ventricular myocytes. J Physiol. 1989 Oct;417:213–239. doi: 10.1113/jphysiol.1989.sp017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. W., Strawbridge R. A., Watanabe A. M. Muscarinic receptor regulation of cardiac adenylate cyclase activity. J Mol Cell Cardiol. 1987 Jan;19(1):47–61. doi: 10.1016/s0022-2828(87)80544-8. [DOI] [PubMed] [Google Scholar]
- Gabev E., Kasianowicz J., Abbott T., McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta. 1989 Feb 13;979(1):105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Effect of forskolin and acetylcholine on calcium current in single isolated cardiac myocytes. Mol Pharmacol. 1987 Nov;32(5):639–645. [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Simmons M. A. Comparison of effects of acetylcholine on calcium and potassium currents in frog atrium and ventricle. J Physiol. 1987 Aug;389:411–422. doi: 10.1113/jphysiol.1987.sp016663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Birnbaumer L. Inhibitory regulation of adenylyl cyclase in the absence of stimulatory regulation. Requirements and kinetics of guanine nucleotide-induced inhibition of the cyc- S49 adenylyl cyclase. J Biol Chem. 1983 Nov 10;258(21):13141–13147. [PubMed] [Google Scholar]
- Hudson T. H., Fain J. N. Forskolin-activated adenylate cyclase. Inhibition by guanyl-5'-yl imidodiphosphate. J Biol Chem. 1983 Aug 25;258(16):9755–9761. [PubMed] [Google Scholar]
- Imoto Y., Yatani A., Reeves J. P., Codina J., Birnbaumer L., Brown A. M. Alpha-subunit of Gs directly activates cardiac calcium channels in lipid bilayers. Am J Physiol. 1988 Oct;255(4 Pt 2):H722–H728. doi: 10.1152/ajpheart.1988.255.4.H722. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Gehring U., Gaugler B., Pfeuffer T., Schultz G. Occurrence of an inhibitory guanine nucleotide-binding regulatory component of the adenylate cyclase system in cyc- variants of S49 lymphoma cells. Eur J Biochem. 1983 Feb 15;130(3):605–611. doi: 10.1111/j.1432-1033.1983.tb07192.x. [DOI] [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Smigel M. D., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and the inhibition of adenylate cyclase in S49 lymphoma cyc- and wild type membranes. J Biol Chem. 1984 Mar 25;259(6):3586–3595. [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Mechanisms for inhibition of the catalytic activity of adenylate cyclase by the guanine nucleotide-binding proteins serving as the substrate of islet-activating protein, pertussis toxin. J Biol Chem. 1986 Apr 15;261(11):5215–5221. [PubMed] [Google Scholar]
- Kemp B. E., Cheng H. C., Walsh D. A. Peptide inhibitors of cAMP-dependent protein kinase. Methods Enzymol. 1988;159:173–183. doi: 10.1016/0076-6879(88)59018-3. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Laurenza A., Sutkowski E. M., Seamon K. B. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci. 1989 Nov;10(11):442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- Martin J. M., Subers E. M., Halvorsen S. W., Nathanson N. M. Functional and physical properties of chick atrial and ventricular GTP-binding proteins: relationship to muscarinic acetylcholine receptor-mediated responses. J Pharmacol Exp Ther. 1987 Feb;240(2):683–688. [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Structural and functional relationships of guanosine triphosphate binding proteins. Curr Top Cell Regul. 1988;29:129–216. doi: 10.1016/b978-0-12-152829-4.50006-9. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Thrombin- and nucleotide-activated phosphatidylinositol 4,5-bisphosphate phospholipase C in human platelet membranes. J Biol Chem. 1987 Apr 25;262(12):5492–5498. [PubMed] [Google Scholar]
- Ross E. M. Signal sorting and amplification through G protein-coupled receptors. Neuron. 1989 Aug;3(2):141–152. doi: 10.1016/0896-6273(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988 Feb 12;239(4841 Pt 1):778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989 Jul 7;245(4913):71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]


