Abstract
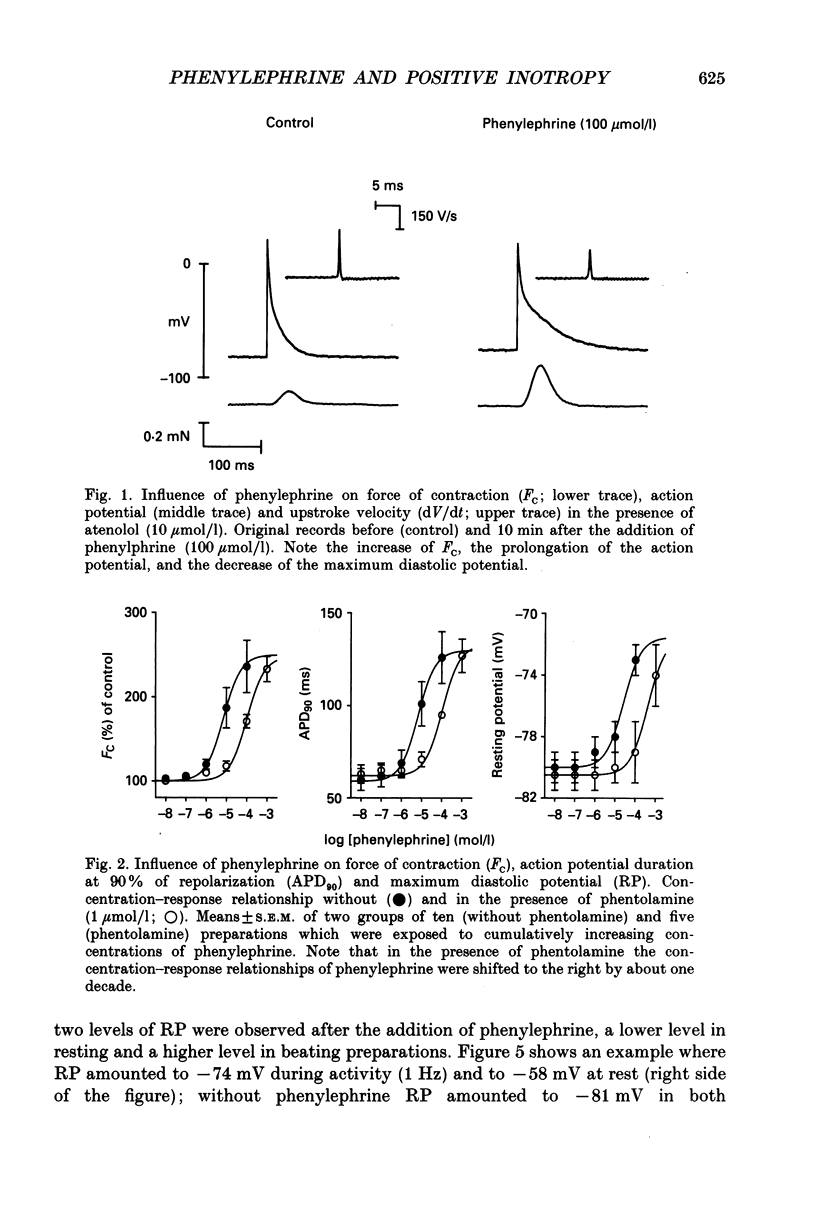
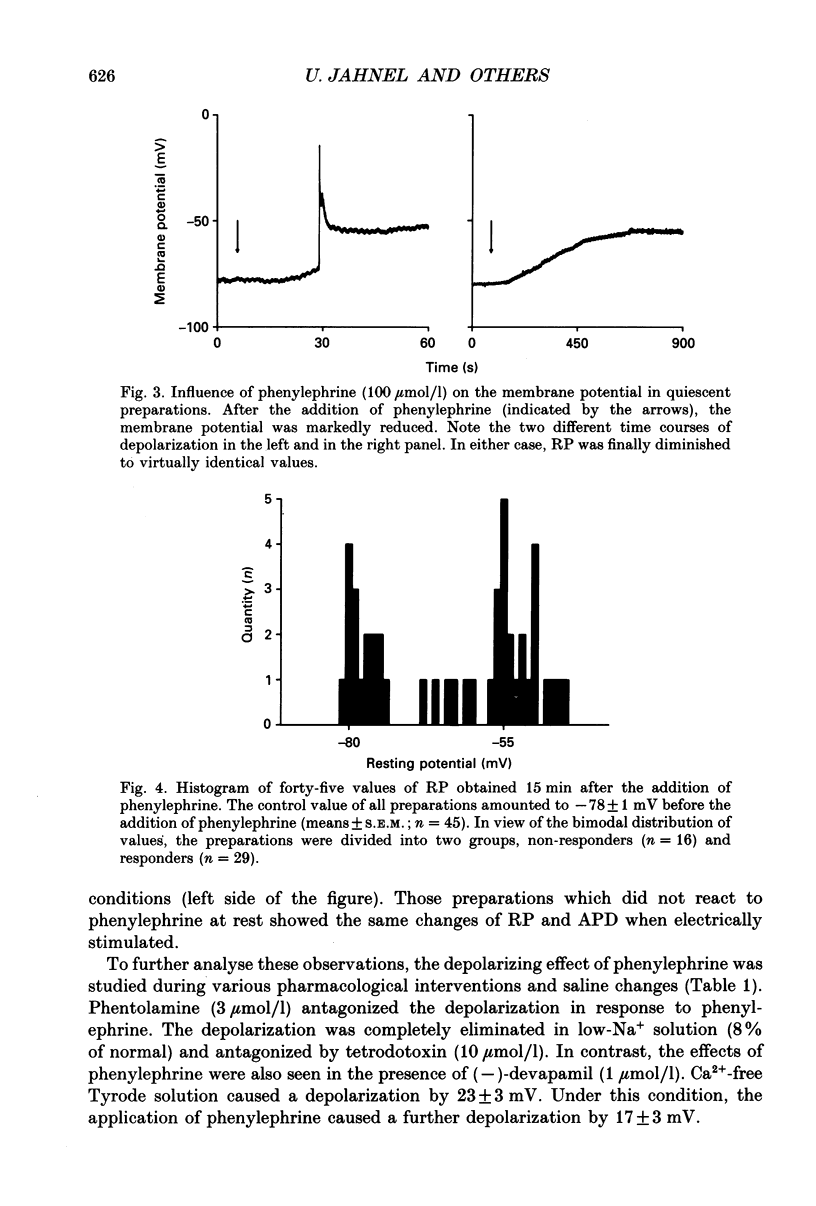
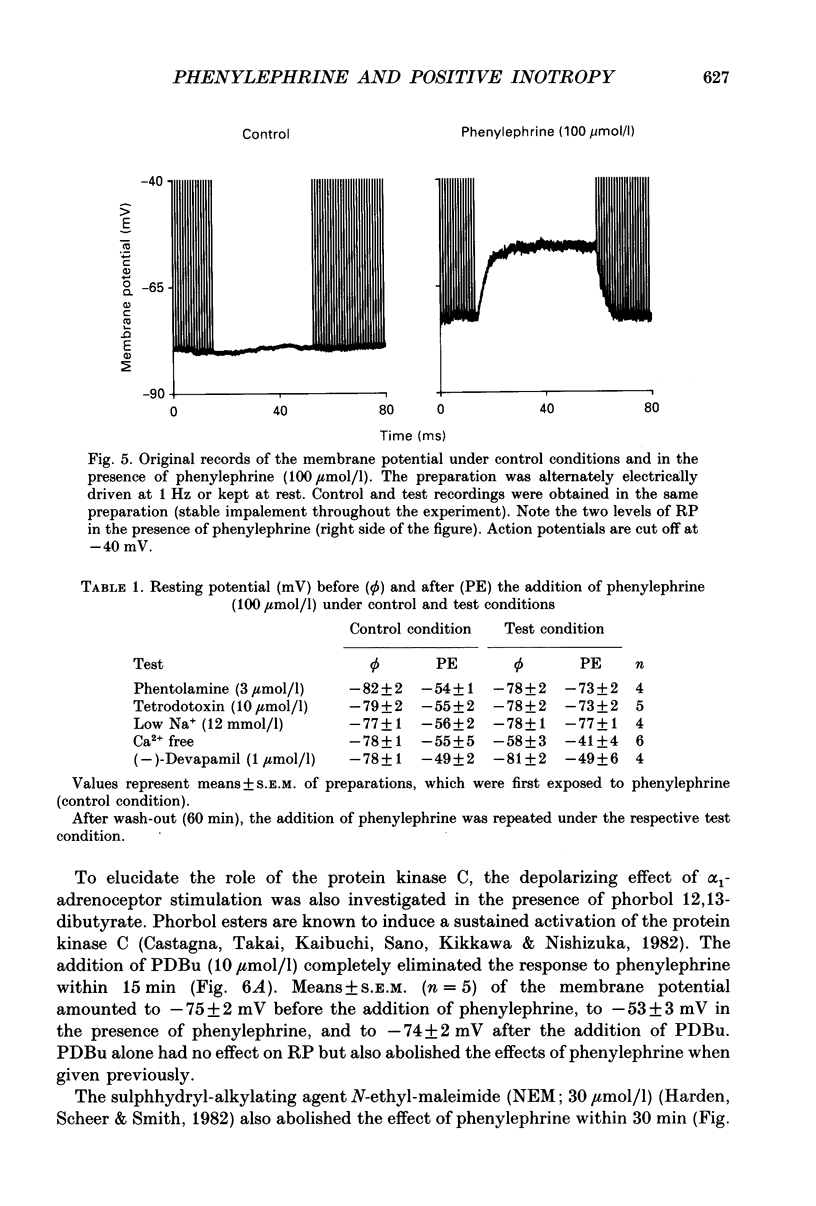
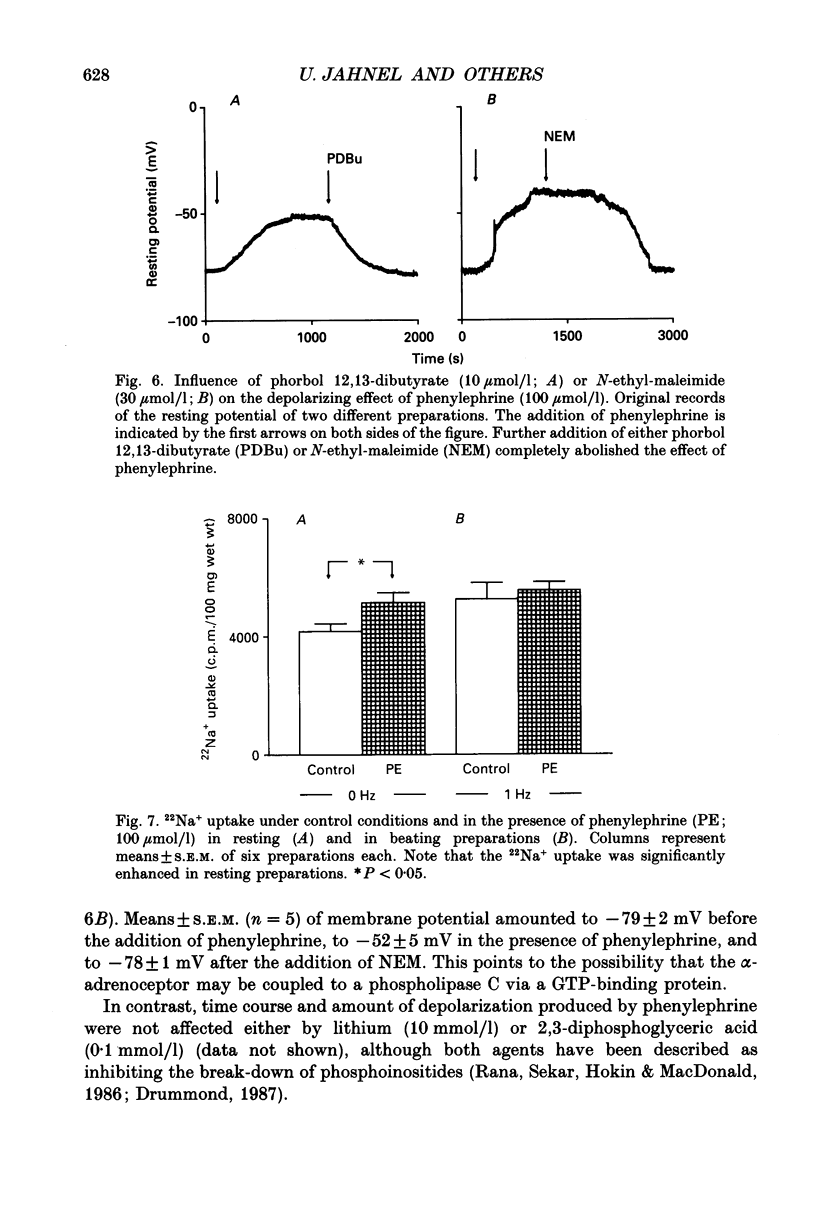
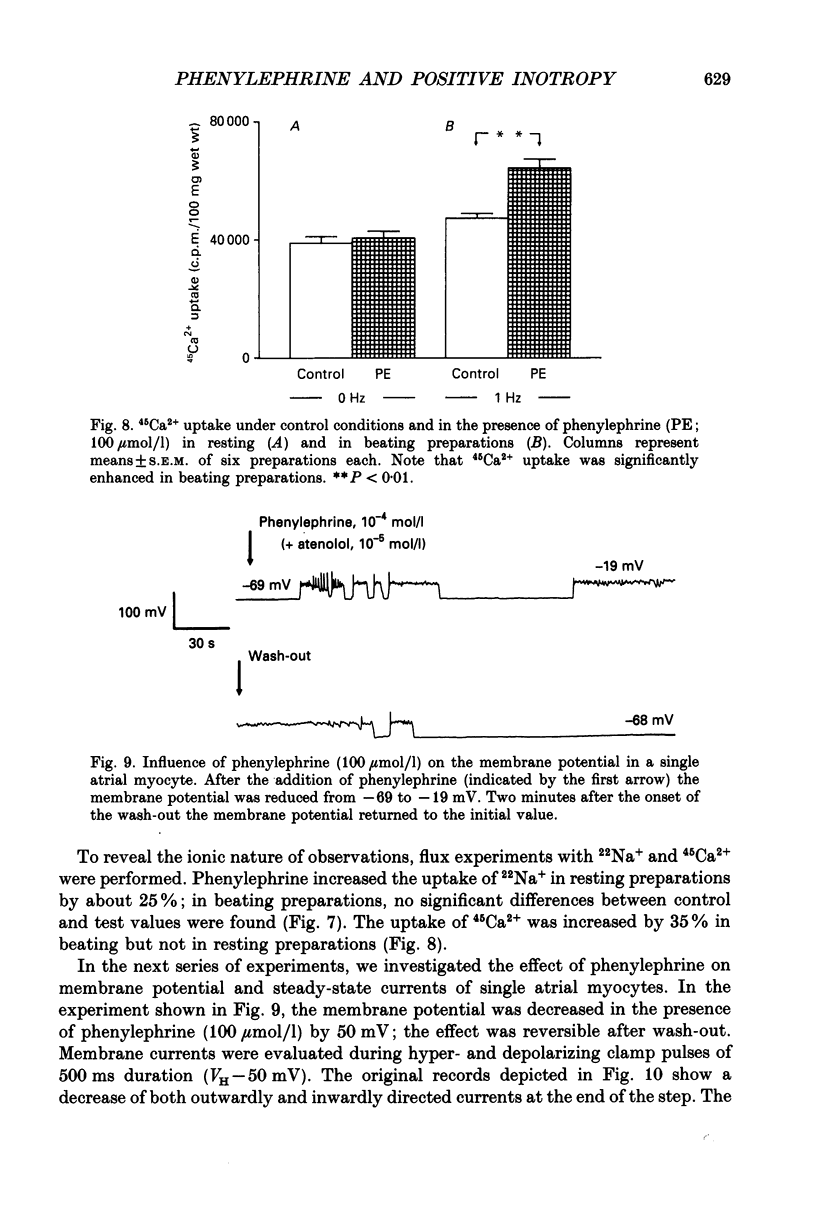
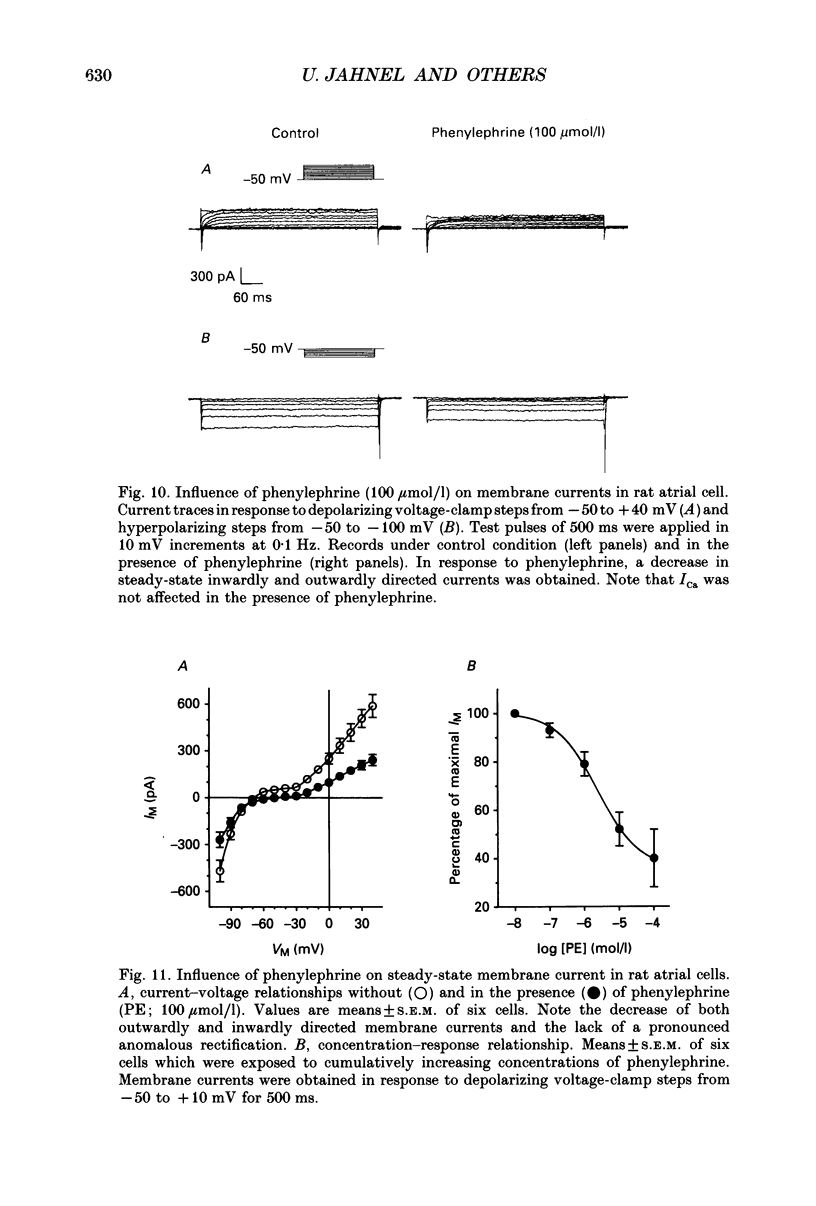
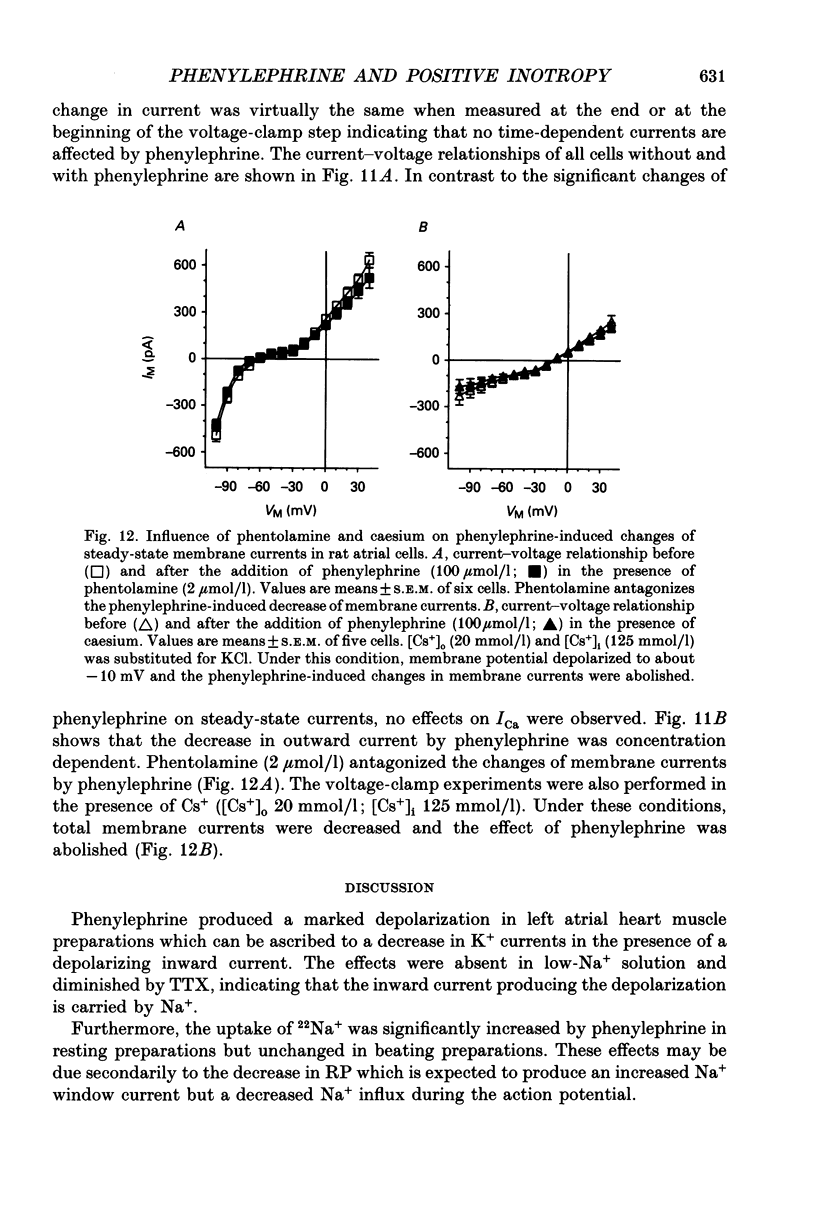
1. The effects of alpha 1-adrenoceptor stimulation on transmembrane potential, currents and ion fluxes were investigated in multicellular preparations and/or single cells obtained from the left atrium of rat hearts. 2. In multicellular preparations, phenylephrine caused a concentration-dependent positive inotropic effect, an increase in action potential duration, and a decrease in resting potential; the effects were antagonized by phentolamine. 3. In the presence of phenylephrine (100 mumol/1), two levels of resting potential were observed when the preparations were, alternately, electrically stimulated or kept at rest (-74 +/- 1 mV during activity and -62 +/- 4 mV at rest; mean +/- S.E.M.; n = 9). 4. In resting preparations, the depolarization in response to phenylephrine was eliminated in low-Na+ solution (12 mmol/l) and antagonized by tetrodotoxin (10 mumol/l). 5. The phenylephrine-induced depolarization was also seen in nominally Ca(2+)-free solution and in the presence of (-)-devapamil (1 mumol/l). 6. The alkylating agent N-ethyl-maleimide (30 mumol/l) abolished the depolarizing effect of phenylephrine. 7. Phorbol 12,13-dibutyrate (10 mumol/l) also abolished the depolarizing effect of phenylephrine. 8. Phenylephrine caused a significant increase of 22Na+ uptake in resting preparations and of 45Ca2+ uptake in beating preparations. 9. The depolarizing effect of phenylephrine was also observed in single atrial myocytes. Steady-state membrane currents in response to 500 ms depolarizing and hyperpolarizing voltage clamp steps were decreased. The cross-over of I-V curves under control and test conditions was at about -70 mV. The effects of phenylephrine were antagonized in the presence of phentolamine. 10. After suppression of potassium currents by substitution of CsCl for internal and external KCl ([KCl]o), phenylephrine had no effect on membrane currents. 11. In conclusion, we presume the following sequence of events in response to phenylephrine in rat atrial heart muscle. First, the stimulation of alpha 1-adrenoceptors decreases the K+ conductance thereby producing a depolarization in the presence of an inward current. Second, the change of the membrane potential in the depolarizing direction induces a TTX-sensitive Na+ window current which further propels the depolarization. Third, the increase in Na+ influx may increase Ca2+ influx by activating the Na(+)-Ca2+ exchange in mechanism. The greater influx of Ca2+ may contribute to the positive inotropic effect in response to phenylephrine.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Apkon M., Nerbonne J. M. Alpha 1-adrenergic agonists selectively suppress voltage-dependent K+ current in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8756–8760. doi: 10.1073/pnas.85.22.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Braun A. P., Sperelakis N. Attenuation by N-ethylmaleimide treatment of the cholinergically induced shortening of action potential duration in guinea pig right atrium. Biochem Pharmacol. 1988 Dec 1;37(23):4577–4581. doi: 10.1016/0006-2952(88)90676-4. [DOI] [PubMed] [Google Scholar]
- Brum G., Flockerzi V., Hofmann F., Osterrieder W., Trautwein W. Injection of catalytic subunit of cAMP-dependent protein kinase into isolated cardiac myocytes. Pflugers Arch. 1983 Jul;398(2):147–154. doi: 10.1007/BF00581064. [DOI] [PubMed] [Google Scholar]
- Brückner R., Scholz H. Effects of alpha-adrenoceptor stimulation with phenylephrine in the presence of propranolol on force of contraction, slow inward current and cyclic AMP content in the bovine heart. Br J Pharmacol. 1984 May;82(1):223–232. doi: 10.1111/j.1476-5381.1984.tb16462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M., Schmitz W., Scholz H. Evidence against a role of a pertussis toxin-sensitive guanine nucleotide-binding protein in the alpha 1-adrenoceptor-mediated positive inotropic effect in the heart. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):476–479. doi: 10.1007/BF00165566. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Corr P. B., Shayman J. A., Kramer J. B., Kipnis R. J. Increased alpha-adrenergic receptors in ischemic cat myocardium. A potential mediator of electrophysiological derangements. J Clin Invest. 1981 Apr;67(4):1232–1236. doi: 10.1172/JCI110139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. I., Severson D. L. Cyclic nucleotides and cardiac function. Circ Res. 1979 Feb;44(2):145–153. doi: 10.1161/01.res.44.2.145. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Endoh M., Blinks J. R. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through alpha- and beta-adrenoceptors. Circ Res. 1988 Feb;62(2):247–265. doi: 10.1161/01.res.62.2.247. [DOI] [PubMed] [Google Scholar]
- Fedida D., Shimoni Y., Giles W. R. A novel effect of norepinephrine on cardiac cells is mediated by alpha 1-adrenoceptors. Am J Physiol. 1989 May;256(5 Pt 2):H1500–H1504. doi: 10.1152/ajpheart.1989.256.5.H1500. [DOI] [PubMed] [Google Scholar]
- Fedida D., Shimoni Y., Giles W. R. Alpha-adrenergic modulation of the transient outward current in rabbit atrial myocytes. J Physiol. 1990 Apr;423:257–277. doi: 10.1113/jphysiol.1990.sp018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamra M., Rosen M. R. Alpha-adrenergic receptor stimulation during simulated ischemia and reperfusion in canine cardiac Purkinje fibers. Circulation. 1988 Dec;78(6):1495–1502. doi: 10.1161/01.cir.78.6.1495. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Scheer A. G., Smith M. M. Differential modification of the interaction of cardiac muscarinic cholinergic and beta-adrenergic receptors with a guanine nucleotide binding component(s). Mol Pharmacol. 1982 May;21(3):570–580. [PubMed] [Google Scholar]
- Hescheler J., Nawrath H., Tang M., Trautwein W. Adrenoceptor-mediated changes of excitation and contraction in ventricular heart muscle from guinea-pigs and rabbits. J Physiol. 1988 Mar;397:657–670. doi: 10.1113/jphysiol.1988.sp017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Ravens U. The effects of the Anemonia sulcata toxin (ATX II) on membrane currents of isolated mammalian myocytes. J Physiol. 1984 Dec;357:127–149. doi: 10.1113/jphysiol.1984.sp015493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. H., Berlin J. R., Ng Y. C., Akera T., Brody T. M. Amiloride: effects on myocardial force of contraction, sodium pump and Na+/Ca2+ exchange. J Mol Cell Cardiol. 1986 Feb;18(2):177–188. doi: 10.1016/s0022-2828(86)80470-9. [DOI] [PubMed] [Google Scholar]
- Korth M., Kühlkamp V. Muscarinic receptor-mediated increase of intracellular Na+-ion activity and force of contraction. Pflugers Arch. 1985 Mar;403(3):266–272. doi: 10.1007/BF00583598. [DOI] [PubMed] [Google Scholar]
- Kushida H., Hiramoto T., Satoh H., Endoh M. Phorbol ester does not mimic, but antagonizes the alpha-adrenoceptor-mediated positive inotropic effect in the rabbit papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):169–176. doi: 10.1007/BF00169245. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann J. P. Alpha-adrenergic stimulation of sarcolemmal protein phosphorylation and slow responses in intact myocardium. J Biol Chem. 1986 Apr 15;261(11):4860–4867. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Pappano A. J. Sodium-dependent membrane current induced by carbachol in single guinea-pig ventricular myocytes. J Physiol. 1989 Aug;415:487–502. doi: 10.1113/jphysiol.1989.sp017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman K. P. Alpha 1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+. Pharmacol Rev. 1988 Jun;40(2):87–119. [PubMed] [Google Scholar]
- Miura Y., Inui J., Imamura H. Alpha-adrenoceptor-mediated restoration of calcium-dependent potential in the partially depolarized rabbit papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jan-Feb;301(3):201–205. doi: 10.1007/BF00507038. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Thomas A. P., Selak M., Williamson J. R. Inositol trisphosphate does not release Ca2+ from permeabilized cardiac myocytes and sarcoplasmic reticulum. FEBS Lett. 1985 Jun 17;185(2):328–332. doi: 10.1016/0014-5793(85)80932-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nosek T. M., Williams M. F., Zeigler S. T., Godt R. E. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C807–C811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- Okumura K., Kawai T., Hashimoto H., Ito T., Ogawa K., Satake T. Sustained diacylglycerol formation in norepinephrine-stimulated rat heart is associated with alpha 1-adrenergic receptor. J Cardiovasc Pharmacol. 1988 Jun;11(6):651–656. doi: 10.1097/00005344-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Otani H., Otani H., Das D. K. Alpha 1-adrenoceptor-mediated phosphoinositide breakdown and inotropic response in rat left ventricular papillary muscles. Circ Res. 1988 Jan;62(1):8–17. doi: 10.1161/01.res.62.1.8. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Sulpice J. C., Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Sekar M. C., Hokin L. E., MacDonald M. J. A possible role for glucose metabolites in the regulation of inositol-1,4,5-trisphosphate 5-phosphomonoesterase activity in pancreatic islets. J Biol Chem. 1986 Apr 25;261(12):5237–5240. [PubMed] [Google Scholar]
- Ravens U., Wang X. L., Wettwer E. Alpha adrenoceptor stimulation reduces outward currents in rat ventricular myocytes. J Pharmacol Exp Ther. 1989 Jul;250(1):364–370. [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974 May;34(5):599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J., Schaefer B., Schmitz W., Scholz H., Steinfath M., Lohse M., Schwabe U., Puurunen J. Alpha-1 adrenoceptor-mediated positive inotropic effect and inositol trisphosphate increase in mammalian heart. J Pharmacol Exp Ther. 1988 Apr;245(1):327–335. [PubMed] [Google Scholar]
- Shah A., Cohen I. S., Rosen M. R. Stimulation of cardiac alpha receptors increases Na/K pump current and decreases gK via a pertussis toxin-sensitive pathway. Biophys J. 1988 Aug;54(2):219–225. doi: 10.1016/S0006-3495(88)82950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonn J. K., Lee C. O. Na+-Ca2+ exchange in regulation of contractility in canine cardiac Purkinje fibers. Am J Physiol. 1988 Sep;255(3 Pt 1):C278–C290. doi: 10.1152/ajpcell.1988.255.3.C278. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. Am J Physiol. 1987 Nov;253(5 Pt 2):H1321–H1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]
- Wagner J., Brodde O. E. On the presence and distribution of alpha-adrenoceptors in the heart of various mammalian species. Naunyn Schmiedebergs Arch Pharmacol. 1978 May;302(3):239–254. doi: 10.1007/BF00508293. [DOI] [PubMed] [Google Scholar]
- Yuan S. H., Sunahara F. A., Sen A. K. Tumor-promoting phorbol esters inhibit cardiac functions and induce redistribution of protein kinase C in perfused beating rat heart. Circ Res. 1987 Sep;61(3):372–378. doi: 10.1161/01.res.61.3.372. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E. A novel cardiac potassium channel that is active and conductive at depolarized potentials. Pflugers Arch. 1988 Dec;413(2):127–133. doi: 10.1007/BF00582522. [DOI] [PubMed] [Google Scholar]


