Abstract
Purpose
Trimodal therapy is a reasonable bladder-preserving option to radical cystectomy. However, many tumors are radioresistive. In this sense, the identification of new prognostic and predictive biomarkers that allow the selection of patients with better responses to radiation therapy would improve outcomes. With the aim of using ecto-5′-nucleotidase/CD73 as a predictive biomarker, the role of this enzyme in the context of radiotherapy in T24 human bladder cancer cell line was investigated.
Methods
T24 cell line was exposure to a single dose of radiation (4 Gray) and trypan blue assay (pharmacological assays of viability/cumulative population doubling), flow cytometry (cell cycle/cell death/active caspase-3/ecto-5′-nucleotidase/CD73 protein staining), DAPI staining (nuclear morphometric assay), RT-PCR and real-time PCR, malachite green method (ectonucleotidase enzymatic assay), and HPLC (analysis of AMP metabolism) were carried out. T24 cell line in which ecto-5′-nucleotidase/CD73 has been completely silenced (5′KO) was also used.
Results
The exposure of T24 cell line to a single dose (4 Gray) of radiation-induced cell death and triggered a transitory increase in ecto-5′-nucleotidase/CD73 expression, increased ectonucleotidase activity, and led to adenosine and inosine accumulation in the extracellular medium. Pharmacological inhibition or knocking out ecto-5′-nucleotidase/CD73 rescued cells’ proliferative capacity, reducing their sensitivity to radiation.
Conclusion
Our findings show that the induction of ecto-5′-nucleotidase/CD73 by radiation contributes to the radiosensitivity of T24 cell line.
Electronic supplementary material
The online version of this article (10.1007/s00432-017-2567-3) contains supplementary material, which is available to authorized users.
Keywords: T24 cell line, Bladder carcinoma, Radiotherapy, Purinergic signaling, Ectonucleotidase
Introduction
Urinary bladder cancer has been estimated to be responsible for approximately 468,400 new cases and 179,800 deaths per year worldwide (GLOBOCAN 2012). Muscle-invasive bladder cancer is defined as occurring when cancer progresses to the muscle layer of the bladder wall, which is a life-threatening condition (Park et al. 2014). For decades, radical cystectomy has been considered the standard treatment for muscle-invasive bladder cancer, but this treatment is associated with significant morbidity and a high complication rate after surgery. Interest in therapeutic approaches aimed at preserving the bladder has emerged as an alternative treatment option (Jacobs et al. 2010; Kotwal et al. 2008; Russell et al. 2016). The trimodality approach has been widely recognized as bladder-preserving therapy, which consists in transurethral resection of bladder tumor (TURBT), chemotherapy, and radiotherapy (Kulkarni et al. 2017; Rödel et al. 2002; Russell et al. 2016). However, many tumors are radioresistive, with radiotherapy being associated with recurrence and distant metastasis, complicating its implementation (James et al. 2012; Park et al. 2014). Therefore, cancer researchers have been greatly interested in identifying prognostic and predictive biomarkers that allow the selection of patients who can avoid radical cystectomy and who require additional radio-sensitization to muscle-invasive bladder cancer treatment (Chen et al. 2013; Shrivastava et al. 2016).
The ecto-5′NT/CD73 enzyme is highly expressed in most solid tumors and has been proposed as a potential clinical or prognostic biomarker (Gao et al. 2014). The expression of ecto-5′NT/CD73 has been described in human bladder cancer cell lines and in cancerous urothelium in a mouse model of bladder tumor induced by BBN (Rockenbach et al. 2014; Stella et al. 2010). The enzyme ecto-5′NT/CD73 is an anchored cell surface protein that participates in extracellular ATP catabolism and is responsible for hydrolyzing AMP to adenosine (Gao et al. 2014; Zimmermann et al. 2012). Ecto-5′NT/CD73 is positively involved in cancer progression, invasion, migration, and adhesion (Bavaresco et al. 2008; Wang et al. 2008). However, this enzyme has also been hypothesized as a favorable prognostic marker in epithelial ovarian cancer, breast cancer, medulloblastoma, and non-invasive urothelial bladder cancer (Cappellari et al. 2015; Oh et al. 2012; Supernat et al. 2012; Wettstein et al. 2015).
Among its previously described functions, the most relevant biological role attributed to ecto-5′NT/CD73 is the extracellular production of adenosine (Gao et al. 2014). Significant levels of extracellular adenosine are present in the tumor microenvironment, modulating a variety of cellular functions by intrinsic and/or extrinsic pathways (Haskó and Cronstein 2004; Merighi et al. 2003). Adenosine may be taken up by nucleoside transporters, either acting intracellularly as a source of de novo synthesis of purine nucleotides or leading cells to apoptosis (Löffler et al. 2007; Mello et al. 2014). Extracellularly, adenosine may act through an interaction with P1 metabotropic receptors (A1, A2A, A2B, and A3) that participate in the control of intracellular cAMP levels (Haskó and Cronstein 2004). Due to the multitude of targets that may be involved in the effect of adenosine on cancer cells, adenosine has been recognized as inducing either cell proliferation (Bavaresco et al. 2008; Zhi et al. 2010) or cell death (Mello et al. 2014; Saito et al. 2010; Shirali et al. 2013; Tsuchiya et al. 2012).
Although signaling involving ecto-5′NT/CD73 and adenosine has an important function in cancer, their role in the response of cancer to radiation therapy is still unknown. In the present study, we identified the importance of the ecto-5′NT/CD73/adenosine pathway at predicting the response of T24 muscle-invasive bladder cancer cell line to radiation treatment. Our findings show that radiation induces a transitory increase in ecto-5′NT/CD73 levels that contributes to radiosensitizing T24 muscle-invasive bladder cancer cell line through adenosine formation.
Materials and methods
Reagents
RPMI-1640, fetal bovine serum (FBS), Fungizone®, penicillin/streptomycin and 0.5% trypsin/EDTA solution were from Gibco Laboratories (Carlsbad, CA, USA). Trypan blue, propidium iodide (PI), all nucleotides and nucleosides, ATPγS, apyrase, tetrabutylammonium chloride, potassium dihydrogen phosphate (KH2PO4), methanol, DMSO, caffeine, dipyridamole and APCP were from Sigma–Aldrich (St Louis, MO, USA). AnnexinV-FITC/PI, active caspase-3 kits, PE mouse anti-human CD73 antibody and PE mouse IgG1 isotype control were from BD Biosciences (Mountain View, CA, USA). DPCPX, SCH 58261, MRS 1754 and EHNA were from Tocris Bioscience (Avonmouth, BS, UK). DAPI was from Calbiochem (San Diego, CA, USA). ABT-702 was from Santa Cruz Biotechnology (Dallas, TX, USA).
Cell culture and treatments
The human bladder cancer cell line T24 was obtained from ATCC (Rockville, MD, USA) and maintained in RPMI culture medium. The medium contained 0.5 U/mL penicillin/streptomycin and 10% FBS. Cells were cultured in a 5% CO2, 95% air atmosphere at 37 °C. The T24 cell line was gamma irradiated at a dose of 2 or 4 Gray (Gy) using Cobalt Theratron Phoenix equipment (Theratronics Ltd, Ontario, Canada) at a source-to-target distance of 54.5 cm. After radiation, the T24 cell line was incubated in a cell incubator for 24, 48 h, 3, or 7 days.
Knockout of ecto-5′NT/CD73
The ecto-5′NT/CD73 knockout was performed in T24 cell line using a CRISPR/Cas system. The following sequence was used to plasmid construction as the target sequence for ecto-5′NT/CD73 knockout: F: 5′-CACCGCAGCACGTTGGGTTCGGCG-3′ and R: 5′-AAACCGCCGAACCCAACGTGCTGC-3′. After cells were transfected with Lipofectamine, clones in which ecto-5′NT/CD73 had been completely silenced (5′KO) were characterized (Supplementary Fig. S1).
Cell counting
T24 cell line was seeded in 24-well plates, grown for 24 h, and then irradiated. After 24 or 48 h, cells were counted in a hemocytometer using the trypan blue dye exclusion test. The results were expressed as the percentage of viable cells in relation to a non-irradiated control group.
Pharmacological assays
The effect of a non-hydrolysable P2 agonist was determined by treating T24 cell line with 100 µM ATPγS for 48 h. To deplete extracellular ATP, 2 U/mL apyrase enzyme (grade VII) was tested in the absence or presence of 1 mM ATP.
To determine the effect of ATP metabolites on T24 cell line death, different concentrations of ATP, ADP, AMP, adenosine (ADO), and inosine (INO) were evaluated for 48 h. UTP and UDP were also evaluated.
To investigate whether extracellular adenosine induced cytotoxicity in T24 cell line, the effect of an adenosine deaminase inhibitor, EHNA, was evaluated. T24 cell line was incubated with 2 µM EHNA, irradiated, and then treated with 1 mM ATP or ADO.
To test whether ATP-induced cell death involves the activation of P1 receptors, we evaluated the effect of antagonists for these receptors. T24 cell line was treated with DMSO (vehicle control), caffeine (30 µM; a non-selective P1 antagonist), DPCPX (10 nM; a selective A1 receptor antagonist), SCH 58261 (50 nM; a selective A2A receptor antagonist), or MRS 1754 (300 nM; a selective A2B receptor antagonist) for 30 min. After this incubation, cells were irradiated and treated with 1 mM ATP.
To evaluate whether ATP was degraded and extracellular adenosine was taken up by the cells, T24 cell line was pretreated with 5 µM dipyridamole for 30 min before radiation, after which 1 mM of ATP or ADO was added for 48 h. To test our hypothesis, the effect of adenosine kinase inhibition was investigated. T24 cell line was pretreated with 100 nM ABT-702 for 30 min before radiation, after which 1 mM of ATP was added for 48 h. ABT-702 was replaced every 24 h.
DMSO was used as a vehicle control at a maximum concentration of 0.5% in the culture medium as indicated. All experiments described in this section were performed on non-irradiated and irradiated T24 cell line with a dose of 4 Gy.
Flow cytometry
All flow cytometry experiments were performed on FACS Calibur equipment (FACS Calibur, BD Bioscience, Mountain View, CA, USA), and the data obtained were analyzed with FLOWJO® software.
Cell cycle and annexin V/PI analysis
T24 cell line was seeded in 6-well plates, grown for 24 h, and irradiated with a dose of 4 Gy. After 24 or 48 h, a cell cycle assay was conducted as previously described (Figueiró et al. 2015), and the results were analyzed by flow cytometry. Apoptotic or necrotic cells were quantified using an Annexin V-FITC/PI double staining kit. After 48 h, the assay was conducted as previously described (Dietrich et al. 2014), and the results were analyzed by flow cytometry.
Active caspase-3 measurement
T24 cell line was seeded in 6-well plates, grown for 24 h, and irradiated with a dose of 4 Gy. After 48 h, the experiment was conducted as previously described (Cappellari et al. 2015), and results were analyzed by flow cytometry.
Ecto-5′NT/CD73 protein staining
T24 cell line was seeded in 6-well plates, grown for 24 h, and irradiated. After 48 h, the experiment was performed. To evaluate ecto-5′NT/CD73 protein staining over time, after 48 h of radiation, cells were reseeded in 6-well plates and grown for 3, 5, and 7 days. In another set of experiments, the mechanism underlying the observed increase in ecto-5′NT/CD73 expression was determined. T24 cell line was treated with DMSO (vehicle control), caffeine (30 µM), SCH 58261 (50 nM), or MRS1754 (100 nM) and then irradiated. After 48 h, the cells were seeded in 6-well plates, once again received treatments and were incubated for 3 days. At the end of both experiments, a total of 3 × 105 cells were centrifuged and incubated with staining buffer [FBS 1% (v/v); sodium azide 0.09% (m/v) in PBS] containing the ecto-5′NT/CD73 PE antibody. The cells were incubated for 30 min on ice in the dark. Isotype control was used as a nonspecific binding control. After incubation, the cells were washed twice and immediately analyzed using flow cytometry.
Nuclear morphometric assay (NMA) and contour plot for NMA
T24 cell line was seeded in 12-well plates, grown for 24 h, and irradiated with a dose of 4 Gy. After 24 h or 7 days, an NMA assay was conducted as previously described (Filippi-Chiela et al. 2012). Data are presented as a plot of area versus nuclear irregularity index (NII). We also present the NMA data depicted as a contour plot graph using SigmaPlot (Systat Software, Inc., San Jose CA, USA).
Cumulative population doubling
To assess cumulative population doubling (CPD), T24 and 5′KO T24 cell line were seeded in 6-well plates and grown for 24 h. T24 cell line was treated with APCP (10 µM) and both cells were irradiated with a dose of 4 Gy. After 48 h, the cells were seeded in 24-well plates and T24 cell line received treatment. After 2, 3, 4, 5, 6, and 7 days in RPMI, the number of cells was counted and the CPD was calculated as previously described (Filippi-Chiela et al. 2015). The sum of the CPDs was plotted versus the duration of culture.
RT-PCR and real-time PCR
T24 cell line was seeded in 6-well plates, grown for 24 h, and irradiated with a dose of 4 Gy. After 48 h, the total RNA from the cell line was isolated with Trizol LS reagent. cDNA was synthesized from 2 µg of total RNA with M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA) in a final volume of 25 µL in the presence of oligo dT primer. An RT-PCR was performed in a total volume of 20 µL, which included 0.75 µL of cDNA, 0.5 µL of each forward and reverse primer (Tables S1, S2), and 10 µL of PCR master mix (Promega Corporation, Madison, WI, USA). The reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. Ten microliters of the RT-PCR were separated on a 2% agarose gel containing SYBR® Safe DNA and visualized under ImageQuant™ LAS 400 (GE Healthcare Life Sciences, Marlborough, MA, USA). Negative control was performed by substituting the templates for DNAse/RNAse-free distilled water in each RT-PCR reaction.
A real-time PCR analysis was performed using an ABI Step One Plus Instrument (Applied Biosystems, Foster City, CA, USA) and a SYBR® Green amplification System. Each reaction was performed in a final volume of 15 µL containing 0.42 µL of cDNA, 7.5 µL of SYBR® Green Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 0.3 µL of each forward and reverse primers (10 µM), and DNAse/RNAse-free distilled water. As the efficiency of all reactions was > 95%, the ΔΔCt parameter was used to determine relative expression data, using GAPDH gene expression as an endogenous control for normalization.
Ectonucleotidase enzymatic assay
To determine the extent of nucleotide hydrolysis, T24 cell line was seeded in 24-well plates, grown for 24 h, and irradiated with a dose of 4 Gy. After 48 h, the differential hydrolysis pattern of ATP, ADP, and AMP was analyzed as previously described (Stella et al. 2010). As a substrate, 2.5 mM of ATP or ADP, or 2 mM of AMP, was used. The incubation proceeded for 30 min. The protein concentration was determined by Coomassie blue staining using bovine serum albumin as a standard (Bradford 1976). The specific activity was expressed as nmol Pi released/min/mg of protein.
Analysis of AMP metabolism by high-pressure liquid chromatography (HPLC)
T24 cell line was seeded in 24-well plates, grown for 24 h, and irradiated with a dose of 4 Gy. After 48 h, the extent of AMP hydrolysis after different incubation times (0, 10, 30, 60, and 90 min) was evaluated as previously described (Figueiró et al. 2016). Protein concentration was determined as described above. Purine concentrations are expressed as nmol product/mg protein.
Statistical analysis
All experiments were performed at least three times. The results are expressed as the means ± SD and were analyzed by one-way ANOVA followed by a Tukey post hoc test, two-way followed by a Bonferroni post hoc test, or by Student’s t test. Differences were considered significant when p < 0.05.
Results
Radiation induces acute G2/M cell cycle arrest and apoptosis in T24 cell line
The T24 cell line is described to be radioresistant at a dose of 2 Gy and radiosensitive at a dose of 4 Gy (Price et al. 2000). As shown in Fig. 1a, we confirmed that a dose of 4 Gy led to a significant reduction in the number of T24 cell line after 24 and 48 h. This reduction in cell number after a dose of 4 Gy was accompanied by a reduction of confluence and changes in cell morphology, with the appearance of enlarged cells and cell fragmentation (Fig. 1b, arrow and arrowhead). After 24 h, radiation increased the number of G2/M cells and hyperdiploid cells, while after 48 h, the cell cycle exhibited a similar profile to that of non-irradiated control cells (Fig. 1c). The cell cycle alterations observed were in agreement with the results found by nuclear morphometric analysis (NMA). 24 h after irradiation, there was an increase in the percentage of cells with large and regular nuclei, a morphological feature of proliferation interference that suggests cell growth arrest (Fig. 1d). 7 days after, we found some irradiated cells with enlarged, regular nuclei, suggesting a senescent phenotype (Fig. 1d, right, blue dashed line circle), some mitotic cells (Fig. 1d, arrow), and most part of irradiated cells presented nuclear morphologies similar to those of control cells, suggesting that T24 cell line that did not die 48 h after irradiation might return to a proliferative state. DAPI staining also showed an increase in cells with micronuclei, a classical marker of acute damage to DNA, mainly 24 h after irradiation (Table S3). We observed that after 48 h radiation triggered apoptosis in 15% of cells, a finding that was corroborated by active caspase-3 measurement (Fig. 1e). Together, these data show that a dose of 4 Gy of radiation primarily causes an early arrest of cell growth and induces apoptosis through the activation of a caspase-3-dependent pathway. However, after a longer period of time, these cells reacquire the phenotypic characteristics of proliferative cells.
Fig. 1.
A dose of 4 Gy of radiation induces cell cycle arrest and apoptosis in T24 cell line. a A cell counting assay after the exposure of T24 cell line to a dose of 2 Gy of radiation for 24 h and a dose of 4 Gy of radiation for 24 and 48 h. ***p < 0.001 in relation to non-irradiated (control) cells as determined by one-way ANOVA, followed by a Tukey’s test. b Representative pictures of non-irradiated and irradiated T24 cell line with a dose of 4 Gy observed under phase-contrast microscopy (×40 magnification). Nuclear increases are indicated by arrows. Suggestions of apoptotic cells are indicated by arrowheads. c The cell cycle distribution after 24 and 48 h. Left panel: representative plots after 24 h. Right: quantification of the percentage of sub-G1, G0/G1, S, G2/M, and hyperdiploid cells at each time point evaluated. d NMA plot of non-irradiated and irradiated T24 cell line after 24 h and 7 days. NII represents the index of nuclear irregularity, as a measurement of nuclear morphometric alterations that occur over time. N normal, Irr irregular, LR large regular, LI large and irregular nuclei. The values on the heat map legends represent numbers of cells. Nuclei from non-irradiated (control) cells are shown in red, while nuclei from irradiated cells are shown in blue; the blue dashed line circle in the 7-day graph represents nuclei from senescent cells; the arrow in the 7-day graph represents nuclei from mitotic cells; e Annexin-V/PI and active caspase-3 flow cytometry assays show that radiation induces apoptosis in T24 cell line after 48 h. Left panel: representative Annexin-V/PI flow cytometry assay showing the average relative number of cells. The gate settings distinguish between early apoptotic (Q1), late apoptotic (Q2), necrotic (Q3), and viable cells (Q4). Right panel: detection of active caspase-3 immunocontent is demonstrated by a histogram that represents non-irradiated (red) and irradiated T24 cell line (blue) and a quantitative analysis. If not specified, T24 cell line was irradiated with a dose of 4 Gy. *p < 0.05, **p < 0.01, ***p < 0.001 in relation to non-irradiated (control) cells as determined by Student’s t test
Radiation-induced cell death is enhanced in the presence of exogenous ATP
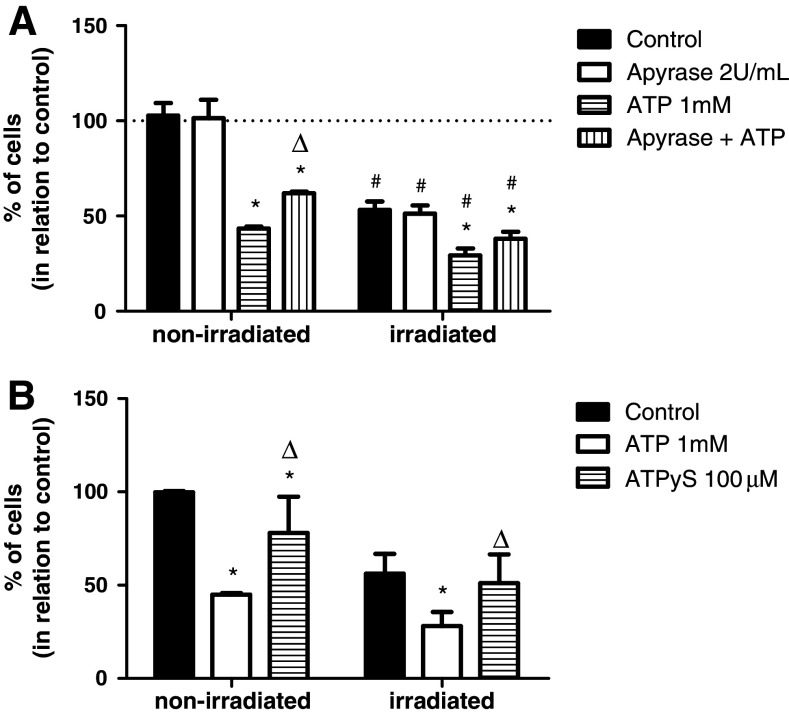
When T24 cell line was treated with 1 mM ATP, the number of non-irradiated cells was significantly reduced by 57%. A dose of 4 Gy of radiation reduces the number of cells by 47% when compared to non-irradiated control cells. A more pronounced reduction in the number of cells (71%) was observed when the cells were exposed to both a dose of 4 Gy of radiation and 1 mM ATP, indicating that T24 cell line is radio- and ATP-sensitive (Fig. 2a). To better investigate the effect of ATP, apyrase was added to treatment and T24 cell line was also exposed to ATPγS, a nondegradable ATP analogue. The most important findings shown in Fig. 2 are: apyrase only partially reverted (20%) the cytotoxic effect of extracellular ATP added to non-irradiated cells (Fig. 2a); and the treatment with ATPγS did not increase the death induced by radiation, indicating that a product of the degradation of ATP, and not ATP per se, might play a role in the cytotoxicity of this nucleotide (Fig. 2b). Altogether, these data suggest that extracellular ATP potentiates the effect of radiation, mainly through a product of its metabolism.
Fig. 2.
Radiation-induced cell death is enhanced in the presence of exogenous ATP. Experiments were performed in non-irradiated and irradiated (dose of 4 Gy) T24 cell line after 48 h. The effects of a exogenous ATP and apyrase, an enzyme that depletes extracellular ATP; and b ATPγS, a nondegradable ATP, were determined by cell counting assays. *Significant difference in relation to respective control; #in relation to respective non-irradiated group; ∆in relation to respective ATP, as determined by two-way ANOVA, followed by Bonferroni’s multiple comparisons test
Radiation induces a transitory up-regulation of ecto-5′NT/CD73, increases ectonucleotidase enzymatic activity, and induces inosine accumulation
To determine whether ectonucleotidases are related to the radiosensitivity of T24 cell line and the production of toxic metabolite derivates, the mRNA levels of ectonucleotidases were analyzed by RT-PCR and real-time PCR. T24 cell line expresses only E-NPP1, NTPDase5, ecto-5′NT/CD73, and adenosine deaminase (ADA) mRNA (Fig. 3a). Real-time PCR analysis demonstrated that a dose of 4 Gy of radiation caused an increase in ecto-5′NT/CD73 mRNA expression in T24 cell line (Fig. 3b). In agreement with real-time PCR data, ecto-5′NT/CD73 protein levels increased after radiation in the T24 cell line (Fig. 3c).
Fig. 3.

Radiation induces a transitory up-regulation of ecto-5′NT/CD73, increases ectonucleotidase enzymatic activity, and induces inosine accumulation. Experiments were performed in non-irradiated and irradiated (dose of 4 Gy) T24 cell line after 48 h. Ectonucleotidases expression were determined by a RT-PCR and b real-time PCR analysis. Expression was normalized to GAPDH signals. c Ecto-5′NT/CD73 expression at the cell surface determined by flow cytometry analysis. The left panel shows a representative histogram of non-irradiated (red) and irradiated T24 cell line (blue). The right panel shows quantitative analysis. d Ectonucleotidases enzymatic activity demonstrates the differential hydrolysis pattern of ATP, ADP, and AMP measured by the malachite green method. e Metabolism of extracellular AMP performed by HPLC analysis; bar graph depicting the average value of inosine plotted in a scatter plot. *p < 0.05, **p < 0.01, ***p < 0.001 in relation to non-irradiated (control) cells as determined by Student’s t test
Although T24 cell line exhibits a low rate of ATP and ADP hydrolysis (Fig. 3d—white and grays bars), a significant increase was observed in irradiated cells after 48 h, suggesting that extracellular ATP and ADP might be hydrolyzed by E-NPP1, which is able to hydrolyze di- and tri-phosphate nucleotides in the extracellular medium. Regarding to AMP hydrolysis, a slight increase was also observed in irradiated cells, suggesting that extracellular AMP might be hydrolyzed by ecto-5′NT/CD73, the main enzyme responsible to hydrolyze monophosphates nucleotides in nucleosides in T24 cell line (Fig. 3d). Then, the AMP metabolism was analyzed by HPLC. Figure 3e and Supplementary Fig. S2 show that AMP was hydrolyzed almost completely after a 60-min incubation in non-irradiated and irradiated T24 cell line. Interesting, in irradiated T24 cell line, inosine accumulated significantly as the main product of AMP metabolism at the end of the incubation.
ADO is the main nucleoside responsible for causing cell death in non-irradiated and irradiated T24 cell line
Thus far, our data suggest that extracellular ATP per se is only partially responsible for the cytotoxicity observed in our experiments, and the main effect of ATP may be explained by the generation of its metabolite derivatives in both non-irradiated and irradiated cells. Exogenous supraphysiological concentrations of ATP, ADP or ADO (1 mM) inhibited cell growth in both non-irradiated and irradiated cells, but only the co-treatment with radiation and either ATP or ADO resulted in an additional reduction in cell number when compared to radiation or nucleotides alone [interaction between radiation and treatments with F(4. 20) = 12.35, p < 0,0001] (Fig. 4a). Other purinergic receptor agonists, such as UTP or UDP (1 mM), did not demonstrate toxic effects in non-irradiated and irradiated T24 cell line (data not shown).
Fig. 4.

ADO is the main nucleoside responsible for causing cell death in non-irradiated and irradiated T24 cell line. Experiments were performed in non-irradiated and irradiated (dose of 4 Gy) T24 cell line after 48 h. a The effect of ATP and ATP metabolites was determined by a cell counting assay. b The effect of EHNA, an adenosine deaminase inhibitor, and inosine (INO) were determined by cell counting assays. P1 receptor expression was determined by c RT-PCR and d real-time PCR analysis. Expression was normalized to GAPDH signals. Cell counting assay after the exposure of T24 cell line to e P1 receptor antagonists, f dipyridamole, an inhibitor of adenosine transport, and g ABT-702, an adenosine kinase inhibitor. a *Significant difference in relation to respective control and #significant difference in relation to respective non-irradiated group; b, f, g * or **significant difference between indicated groups as determined by two-way ANOVA, followed by Bonferroni’s multiple comparisons test. To figure d Student’s t test was used
To investigate whether the observed cytotoxic effect on T24 cell line is induced exclusively by extracellular ADO, T24 cell line was incubated with 1 mM ATP or ADO in the absence or presence of EHNA (an adenosine deaminase inhibitor). Figure 4b shows that EHNA significantly increased the cytotoxic effect of ADO in both non-irradiated and irradiated cells, indicating that this effect was mediated by accumulated ADO, and not by its metabolite inosine. Confirming this finding, treatment with exogenous inosine was not cytotoxic in T24 cell line (Fig. 4b). These data support the conclusion that ADO is the main nucleoside responsible for causing cell death triggered by extracellular ATP in non-irradiated and irradiated T24 cell line.
RT-PCR and real-time PCR analysis confirmed that T24 cell line expresses only A1, A2A, and A2B adenosine receptors, and no alteration of the mRNA expression of these receptors was observed after a dose of 4 Gy of radiation treatment (Fig. 4c, d). We also found that none of the selective antagonists of P1 receptors blocked ATP-induced cell death (Fig. 4e). Therefore, it is possible to conclude that the cytotoxic effect of ADO (generated by ATP hydrolysis) is not exerted by extracellular ADO in either non-irradiated or irradiated T24 cell line, suggesting that ADO may act inside these cells.
We treated the cells with ATP and ADO in the presence of dipyridamole, an inhibitor of adenosine transport. We found that dipyridamole inhibited the cell death induced by ATP and ADO, suggesting that the observed cytotoxic effect might be exerted by intracellular ADO in non-irradiated cells. These results support the hypothesis that ADO uptake is primarily responsible for cell death induced by extracellular ATP or ADO in T24 bladder cancer cell line. In irradiated cells, dipyridamole did not affect ATP or ADO-induced cytotoxicity (Fig. 4f). Likewise, the inhibition of adenosine kinase, the enzyme that converts ADO to AMP intracellularly, by ABT-702 completely blocked the induction of cell death by ATP in non-irradiated cells, showing that the intracellular phosphorylation of ADO to AMP is a key step in the toxicity of exogenous ATP treatment. In irradiated cells, the cytotoxicity induced by ATP was not affected (Fig. 4g).
Radiation induces a transitory increase in ecto-5′NT/CD73 levels
As demonstrated above, ecto-5′NT/CD73 expression is increased 48 h after radiation (Fig. 3b, c). To determine the relationship between the observed increase in ecto-5′NT/CD73 expression and radiation, we used flow cytometry to determine the levels of this enzyme in cells after treatment with radiation for 7 days, as shown in Fig. 5a. The cells that survived a dose of 4 Gy of radiation exhibited increased ecto-5′NT/CD73 protein levels, with the highest expression levels achieved on day 3. On day 7, ecto-5′NT/CD73 protein levels decreased to the same expression level as observed 48 h after radiation (Fig. 5b, day zero).
Fig. 5.
Ecto-5′NT/CD73 contributes to radiation toxicity. a Schematic representation of the experimental setup. b Ecto-5′NT/CD73 expression on the cell surface was determined by flow cytometry analysis on days 3, 5, and 7. The left panel shows a representative histogram of isotype control (green) and irradiated T24 cell line after 3 (red), 5 (blue), and 7 days (orange). The right panel shows a quantitative analysis plotted using a scatter plot where zero corresponds to ecto-5′NT/CD73 protein levels after 48 h. Irradiated cells after 3D, 5D, and 7D were analyzed in relation to their respective controls (3D, 5D, and 7D). Control cells from the respective days exhibited similar expression values. c Ecto-5′NT/CD73 expression was determined by flow cytometry analysis on day 3 after treatment with unspecific P1 and specific A2A and A2B receptor antagonists. #Significant difference in relation to non-irradiated respective group as determined by two-way ANOVA, followed by Bonferroni’s multiple comparisons test. d Cumulative population doubling (CPD) of T24, T24 treated with APCP, and T24 5′KO cell line was determined as shown in (a). *p < 0.05, **p < 0.01, ***p < 0.001 in relation to non-irradiated control, APCP, or 5′KO cells as determined by Student’s t test. Bar graph depicting average values of CPD on day 3 plotted as a scatter plot. **p < 0.01, in relation to irradiated control cells as determined by one-way ANOVA followed by a Tukey’s test
In an attempt to understand the mechanism by which ecto-5′NT/CD73 expression is increased in T24 cell line after exposure to radiation, the cells were treated with unspecific P1 (caffeine) and specific A2A (SCH 58261) and A2B (MRS 1754) antagonists. The results, presented in Fig. 5c, show that caffeine and SCH 58261 did not reverse the effects induced by radiation. However, MRS 1754 decreased the effects of radiation on the expression of ecto-5′NT/CD73 induced by a dose of 4 Gy of radiation. Although there were no significant differences observed between the MRS 1754 and DMSO irradiated groups, it is possible to infer that the observed increase in ecto-5′NT/CD73 expression may be regulated by A2B receptors, as the MRS 1754 group did not differ from the non-irradiated group.
To understand the outcomes of irradiated cells after injury and over time, we evaluated cumulative population doubling (CPD) over 7 days. Figure 5d shows that T24 cell line proliferation was reduced until 3 days after radiation, which coincided with an increase in the expression of ecto-5′NT/CD73 protein. After this period of high expression, the cells proliferated at a ratio similar to that of control cells, which corroborates NMA data (Fig. 1d) showing that, after 7 days, nuclei from irradiated cells exhibited normal phenotypes including mitotic figures. Importantly, the 5′KO cells and cells treated with APCP rescue, at least partially, irradiated cells from death. While T24 cell line reached their lowest CPD 3 days after radiation, the population of APCP-treated T24 and 5′KO cell line had already doubled once 3 days after radiation (Fig. 5d). Thus far, our data confirm that ecto-5′NT/CD73 contributes to radiation toxicity, and its suppression protects cells from the toxicity of radiation when evaluated over a long term.
Discussion
Cancer cells exposed to radiation or other cytotoxic cancer therapies have two opposing cell fates: life or death (Huang et al. 2011). In this study, we showed that a dose of 4 Gy of radiation reduced the numbers of T24 invasive urothelial bladder cancer cells mainly by cell cycle arrest and the induction of apoptosis. Over a long term, T24 cell line returns to proliferative rates similar to those of control cells; this confirms earlier studies showing that a few cells are able to proliferate and re-establish a tumor after exposure to radiation (Hermens and Barendsen 1969; Huang et al. 2011; Stephens et al. 1978).
In this study, we show that the inhibition of the cell growth of non-irradiated and irradiated T24 cell line by extracellular ATP is induced mainly by a product of the degradation of this nucleotide. Furthermore, the addition of ATP or ADO induces greater toxicity when presented in conjunction with radiation, likely acting through a separate route. T24 cell line expresses some ectonucleotidases and can hydrolyze mono-, di- and tri-phosphate nucleotides in the extracellular medium. This suggests that extracellular ATP can be hydrolyzed by the combined action of E-NPP1 and ecto-5′NT/CD73 ectoenzymes, producing the cytotoxic metabolite ADO in both non-irradiated and irradiated cells.
Adenosine has a dual role and can stimulate as well as inhibit tumor cell growth depending on the expression profile of P1 receptors and the cell type involved (Gessi et al. 2011). Although T24 cell line expresses A1, A2A, and A2B adenosine receptors, the specific antagonists of these receptors were ineffective at blocking radiation-induced cell death. Therefore, our results suggest the involvement of intracellular ADO in T24 cell line death. In non-irradiated cells, the mechanism by which ADO leads to cell death is primarily ADO uptake and its consequent phosphorylation to AMP by adenosine kinase. The equilibrative nucleoside transporter inhibitor dipyridamole, as well as the adenosine kinase inhibitor ABT-702, were able to block almost all ATP-induced cell death in T24 cell line.
We also demonstrated that radiation promotes the up-regulation of ecto-5′NT/CD73 in T24 cell line. This enzyme has been described to be up-regulated in radioresistant esophageal cancer cell lines (Fukuda et al. 2004) and in fibroses developed after thoracic radiation (Wirsdörfer et al. 2016). Irradiated T24 cell line with higher ecto-5′NT/CD73 expression levels additionally exhibited decreased proliferation rates. These results strengthen our hypothesis that ADO, formed by the action of up-regulated ecto-5′NT/CD73, potentiates radiation-induced cell death in T24 cell line (Fig. 6). This hypothesis was confirmed by results obtained at a basal level when an inhibitor of ecto-5′NT/CD73, as well as a knockout of ecto-5′NT/CD73, were studied. These data indicate that the absence or the inhibition of ecto-5′NT/CD73 rescues the proliferative capacity of cells due the inability of these cell lines to accumulate ADO in response to radiation. We observed that the increase in ecto-5′NT/CD73 expression in irradiated cells may be regulated by the A2B receptor and is likely also regulated by the generation of extracellular ADO. ADO, by binding to the A2B receptor, may lead to an increase in cAMP levels and CREB activation, with the consequence of regulating ecto-5′NT/CD73 expression (Narravula et al. 2000; Synnestvedt et al. 2002). To prove the proposed relevance of ecto-5′NT/CD73 in radiotherapy treatment would require in vivo experiments using our established mouse model (Rockenbach et al. 2014).
Fig. 6.

Schematic illustration of how the uptake of adenosine induces T24 cell line death in response to radiation (4 Gy). Radiation induces cell death in T24 cell line by cell cycle arrest and the induction of apoptosis (Fig. 1). Radiation induces the up-regulation of ecto-5′NT/CD73 protein levels (Fig. 3a–c) and activity (Fig. 3d), thereby producing ADO, which potentiates radiation-induced cell death in T24 cell line by ADO uptake (Fig. 4f). Intracellularly, ADO can be phosphorylated to AMP by adenosine kinase (ADK). We suggest that intracellular AMP leads to T24 cell line death via dATP accumulation and AMPK phosphorylation, as was previously shown in other cancer cells (Mello et al. 2014). Either an adenosine transporter inhibitor (DIP—dipyridamole) (Fig. 4f) or an adenosine kinase inhibitor (ABT-702) (Fig. 4g) completely block the induction of cell death. Therefore, we suggest that a portion of the generated extracellular ADO is taken up by the cells and a portion generates extracellular INO through ecto-adenosine deaminase (ADA). INO, in turn, may accumulate in the extracellular medium and protect cells from radiation, likely by triggering adenosine receptors (A1 or A2A) or by competing with ADO at nucleoside transporters (Haskó et al. 2004). → stimulation, –| inhibition, ---> hypothesis, ADP adenosine 5′-diphosphate, AMP adenosine 5′-monophosphate, ADO adenosine, INO inosine, dADP deoxyadenosine 5′-diphosphate, dATP deoxyadenosine 5′-triphospate, CD73 ecto-5′NT/CD73, NT nucleoside transporter
Although inosine reached the highest levels of any metabolite in the extracellular medium surrounding irradiated cells, we have provided evidence that inosine does not induce cytotoxic effects in either non-irradiated or irradiated T24 cell line. Indeed, inosine has been described as a radioprotective agent effective at preventing oxidative damage to DNA and decreasing ROS generation in vitro (Gudkov et al. 2006) and significantly increases the survival rates of mice after radiation (Gudkov et al. 2006; Hou et al. 2007; Popova et al. 2014). We suggest that the accumulation of inosine 48 h after radiation may contribute to suppressing radiation-induced cell death. This may explain why T24 cell line can resume proliferation days after radiation. Thus, the generated ADO is partially taken up by the cells and partially used to generate INO in the extracellular medium. INO, in turn, may accumulate in the extracellular medium and protect cells from radiation, likely by triggering adenosine receptors (A1 or A2A) or by competing with ADO at nucleoside transporters (Haskó et al. 2004). Therefore, ADO and INO appear to have opposite actions on T24 cell line: ADO induces cell death, and INO protects cells from radiation.
Studies involving patient samples have revealed that the overexpression of ecto-5′NT/CD73 is associated with a good prognosis in cases of breast cancer (Supernat et al. 2012) and epithelial ovarian cancer (Oh et al. 2012). In medulloblastoma, the inhibition of ecto-5′NT/CD73 promoted cell proliferation in vitro, and its overexpression led to a reduction in tumor growth in vivo (Cappellari et al. 2015). We found that high ecto-5′NT/CD73 expression induced by radiation in bladder cancer cells significantly radiosensitizes T24 cell line, leading to slower growing tumors in vitro. To our knowledge, this is the first study that proposes the investigation of the role of ecto-5′NT/CD73 in the context of radiotherapy used to treat bladder cancer disease. Although more investigations are necessary, especially clinical trials, our in vitro study suggests that ecto-5′NT/CD73 might be a potential marker to predict the efficacy of radiotherapeutic response in bladder cancer treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank to all the São Lucas Hospital Radiotherapy team (SERP, Hospital São Lucas de Porto Alegre). We thank also to Guido Lenz (UFRGS) and Diogo Onofre Gomes de Souza (UFRGS) for kindly provide ABT-702, DAPI and EHNA, respectively.
Abbreviations
- ecto-5′NT/CD73
Ecto-5′-nucleotidase/CD73
- Gy
Gray
- PI
Propidium iodide
- ATPγS
Adenosine 5′-O-(3 thiotriphosphate)
- DMSO
Dimethyl sulfoxide
- APCP
Adenosine 5′-(α,β,methylene)diphosphate
- DPCPX
8-Cyclopentyl-1,3-dipropylxanthine
- SCH 58261
2-(2-Furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine
- MRS 1754
N-(4-Cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide
- DAPI
4′,6-Diamidino-2-phenylindole
- EHNA
erythro-9-(2-Hydroxy-3-nonyl)adenine hydrochloride
- ABT-702
4-Amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido[2,3-d]pyrimidine
- 5′KO
T24 cell line completely silenced to ecto-5′-nucleotidase/CD73
- ADO
Adenosine
- INO
Inosine
- NMA
Nuclear morphometric assay
- NII
Nuclear irregularity index
- CPD
Cumulative population doubling
- HPLC
High-pressure liquid chromatography
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), CNPq-Brazil (Project No 303264/2013-6), FAPERGS/PRONEX (Project number: 16/2551-0000473-0), INCT-2014 (Project 465671/2014-4) and Fundo de Incentivo a Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE/HCPA). FD and PBP were recipients of CNPq fellowships. LR was recipient of Capes fellowship. FF, ARC and ECFC are recipient of PNPD/Capes fellowships. JS was supported by grants from the Canadian Institutes of Health Research. JS is also a recipient of a “Chercheur National” Scholarship award from the “Fonds de recherche du Québec-Santé”.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Bavaresco L, Bernardi A, Braganhol E, Cappellari AR, Rockenbach L, Farias PF, Wink MR, Delgado-Cañedo A, Battastini AM (2008) The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem 319(1–2):61–68. 10.1007/s11010-008-9877-3 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Cappellari AR, Pillat MM, Souza HD, Dietrich F, Oliveira FH, Figueiró F, Abujamra AL, Roesler R, Lecka J, Sévigny J, Battastini AM, Ulrich H (2015) Ecto-5′-nucleotidase overexpression reduces tumor growth in a xenograph medulloblastoma model. PLoS One 10(10):e0140996. 10.1371/journal.pone.0140996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RC, Shipley WU, Efstathiou JA, Zietman AL (2013) Trimodality bladder preservation therapy for muscle-invasive bladder cancer. J Natl Compr Cancer Netw 11(8):952–960 [DOI] [PubMed] [Google Scholar]
- Dietrich F, Kaiser S, Rockenbach L, Figueiró F, Bergamin LS, da Cunha FM, Morrone FB, Ortega GG, Battastini AM (2014) Quinovic acid glycosides purified fraction from Uncaria tomentosa induces cell death by apoptosis in the T24 human bladder cancer cell line. Food Chem Toxicol 67:222–229. 10.1016/j.fct.2014.02.037 [DOI] [PubMed] [Google Scholar]
- Figueiró F, de Oliveira CP, Rockenbach L, Mendes FB, Bergamin LS, Jandrey EH, Edelweiss MI, Guterres SS, Pohlmann AR, Battastini AM (2015) Pharmacological improvement and preclinical evaluation of methotrexate-loaded lipid-core nanocapsules in a glioblastoma model. J Biomed Nanotechnol 11(10):1808–1818 [DOI] [PubMed] [Google Scholar]
- Figueiró F, de Oliveira CP, Bergamin LS, Rockenbach L, Mendes FB, Jandrey EH, Moritz CE, Pettenuzzo LF, Sévigny J, Guterres SS, Pohlmann AR, Battastini AM (2016) Methotrexate up-regulates ecto-5′-nucleotidase/CD73 and reduces the frequency of T lymphocytes in the glioblastoma microenvironment. Purinergic Signal 12(2):303–312. 10.1007/s11302-016-9505-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi-Chiela EC, Oliveira MM, Jurkovski B, Callegari-Jacques SM, da Silva VD, Lenz G (2012) Nuclear morphometric analysis (NMA): screening of senescence, apoptosis and nuclear irregularities. PLoS One 7(8):e42522. 10.1371/journal.pone.0042522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi-Chiela EC, Bueno e Silva MM, Thomé MP, Lenz G (2015) Single-cell analysis challenges the connection between autophagy and senescence induced by DNA damage. Autophagy 11(7):1099–1113. 10.1080/15548627.2015.1009795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Sakakura C, Miyagawa K, Kuriu Y, Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y, Yamagishi H (2004) Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer 91(8):1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZW, Dong K, Zhang HZ (2014) The roles of CD73 in cancer. Biomed Res Int. 10.1155/2014/460654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA (2011) Adenosine receptors and cancer. Biochim Biophys Acta 1808(5):1400–1412. 10.1016/j.bbamem.2010.09.020 [DOI] [PubMed] [Google Scholar]
- GLOBOCAN (2012) Estimated cancer incidence, mortality and Prevalence worldwide in 2012: International Agency for Research on Cancer website. http://globocan.iarc.fr. Accessed 15 May 2016
- Gudkov SV, Shtarkman IN, Smirnova VS, Chernikov AV, Bruskov VI (2006) Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radioprotectors in mice. Radiat Res 165(5):538–545 [DOI] [PubMed] [Google Scholar]
- Haskó G, Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25(1):33–39 [DOI] [PubMed] [Google Scholar]
- Haskó G, Sitkovsky MV, Szabó C (2004) Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci 25(3):152–157 [DOI] [PubMed] [Google Scholar]
- Hermens AF, Barendsen GW (1969) Changes of cell proliferation characteristics in a rat rhabdomyosarcoma before and after x-irradiation. Eur J Cancer 5(2):173–189 [DOI] [PubMed] [Google Scholar]
- Hou B, Xu ZW, Yang CW, Gao Y, Zhao SF, Zhang CG (2007) Protective effects of inosine on mice subjected to lethal total-body ionizing irradiation. J Radiat Res 48(1):57–62 [DOI] [PubMed] [Google Scholar]
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, O’Sullivan B, He Z, Peng Y, Tan AC, Zhou L, Shen J, Han G, Wang XJ, Thorburn J, Thorburn A, Jimeno A, Raben D, Bedford JS, Li CY (2011) Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 17(7):860–866. 10.1038/nm.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Lee CT, Montie JE (2010) Bladder cancer in 2010: how far have we come? CA Cancer J Clin 60(4):244–272. 10.3322/caac.20077 [DOI] [PubMed] [Google Scholar]
- James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA, BC2001 Investigators (2012) Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366(16):1477–1488. 10.1056/NEJMoa1106106 [DOI] [PubMed] [Google Scholar]
- Kotwal S, Choudhury A, Johnston C, Paul AB, Whelan P, Kiltie AE (2008) Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys 70(2):456–463 [DOI] [PubMed] [Google Scholar]
- Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, Bostrom PJ, Kuk C, Li K, Templeton AJ, Sridhar SS, van der Kwast TH, Chung P, Bristow RG, Milosevic M, Warde P, Fleshner NE, Jewett MAS, Bashir S, Zlotta AR (2017) Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol 35(20):2299–2305. 10.1200/JCO.2016.69.2327 [DOI] [PubMed] [Google Scholar]
- Löffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK (2007) Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol 27(5):1004–1013 [DOI] [PubMed] [Google Scholar]
- Mello P, de A, Filippi-Chiela, Nascimento EC, Beckenkamp J, Santana A, Kipper DB, Casali F, Nejar Bruno EA, Paccez A, Zerbini JD, Wink LF, Lenz MR, Buffon G A (2014) Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol Biol Cell 25(19):2905–2918. 10.1091/mbc.E14-01-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Mirandola P, Varani K, Gessi S, Leung E, Baraldi PG, Tabrizi MA, Borea PA (2003) A glance at adenosine receptors: novel target for antitumor therapy. Pharmacol Ther 100(1):31–48 [DOI] [PubMed] [Google Scholar]
- Narravula S, Lennon PF, Mueller BU, Colgan SP (2000) Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol 165(9):5262–5268 [DOI] [PubMed] [Google Scholar]
- Oh HK, Sin JI, Choi J, Park SH, Lee TS, Choi YS (2012) Overexpression of CD73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory T cell infiltration. J Gynecol Oncol 23(4):274–281. 10.3802/jgo.2012.23.4.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC, Citrin DE, Agarwal PK, Apolo AB (2014) Multimodal management of muscle-invasive bladder cancer. Curr Probl Cancer 38(3):80–108. 10.1016/j.currproblcancer.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NR, Gudkov SV, Bruskov VI (2014) Natural purine compounds as radioprotective agents. Radiats Biol Radioecol 54(1):38–49 [PubMed] [Google Scholar]
- Price ME, McKelvey-Martin VJ, Robson T, Hirst DG, McKeown SR (2000) Induction and rejoining of DNA double-strand breaks in bladder tumor cells. Radiat Res 153(6):788–794 [DOI] [PubMed] [Google Scholar]
- Rockenbach L, Braganhol E, Dietrich F, Figueiró F, Pugliese M, Edelweiss MI, Morrone FB, Sévigny J, Battastini AM (2014) NTPDase3 and ecto-5′-nucleotidase/CD73 are differentially expressed during mouse bladder cancer progression. Purinergic Signal 10(3):421–430. 10.1007/s11302-014-9405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel C, Grabenbauer GG, Kühn R, Papadopoulos T, Dunst J, Meyer M, Schrott KM, Sauer R (2002) Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol 20(14):3061–3071 [DOI] [PubMed] [Google Scholar]
- Russell CM, Lebastchi AH, Borza T, Spratt DE, Morgan TM (2016) The role of transurethral resection in trimodal therapy for muscle-invasive bladder cancer. Bladder Cancer 2(4):381–394. 10.3233/BLC-160076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Yaguchi T, Yasuda Y, Nakano T, Nishizaki T (2010) Adenosine suppresses CW2 human colonic cancer growth by inducing apoptosis via A(1) adenosine receptors. Cancer Lett 290(2):211–215. 10.1016/j.canlet.2009.09.011 [DOI] [PubMed] [Google Scholar]
- Shirali S, Aghaei M, Shabani M, Fathi M, Sohrabi M, Moeinifard M (2013) Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumour Biol 34(2):1085–1095. 10.1007/s13277-013-0650-1 [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Mansure JJ, Almajed W, Cury F, Ferbeyre G, Popovic M, Seuntjens J, Kassouf W (2016) The role of HMGB1 in radioresistance of bladder cancer. Mol Cancer Ther 15(3):471–479. 10.1158/1535-7163.MCT-15-0581 [DOI] [PubMed] [Google Scholar]
- Stella J, Bavaresco L, Braganhol E, Rockenbach L, Farias PF, Wink MR, Azambuja AA, Barrios CH, Morrone FB, Oliveira Battastini AM (2010) Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol 28(3):260–267. 10.1016/j.urolonc.2009.01.035 [DOI] [PubMed] [Google Scholar]
- Stephens TC, Currie GA, Peacock JH (1978) Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. Br J Cancer 38(5):573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supernat A, Markiewicz A, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J, Sejda A, Szade J, Czapiewski P, Biernat W, Zaczek A (2012) CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol 20(2):103–107 [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP (2002) Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110(7):993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya A, Kanno T, Saito M, Miyoshi Y, Gotoh A, Nakano T, Nishizaki T (2012) Intracellularly transported adenosine induces apoptosis in MCF-7 human breast cancer cells by accumulating AMID in the nucleus. Cancer Lett 321(1):65–72. 10.1016/j.canlet.2012.02.023 [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, Yin L, Shao Z, Ou Z, Zhou P (2008) Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol 134(3):365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein MS, Buser L, Hermanns T, Roudnicky F, Eberli D, Baumeister P, Sulser T, Wild P, Poyet C (2015) CD73 predicts favorable prognosis in patients with nonmuscle-invasive urothelial bladder cancer. Dis Markers 2015:785461. 10.1155/2015/785461 [DOI] [PMC free article] [PubMed]
- Wirsdörfer F, de Leve S, Cappuccini F, Eldh T, Meyer AV, Gau E, Thompson LF, Chen NY, Karmouty-Quintana H, Fischer U, Kasper M, Klein D, Ritchey JW, Blackburn MR, Westendorf AM, Stuschke M, Jendrossek V (2016) Extracellular adenosine production by ecto-5′-nucleotidase (CD73) enhances radiation-induced lung fibrosis. Cancer Res 76(10):3045–3056. 10.1158/0008-5472.CAN-15-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi X, Wang Y, Zhou X, Yu J, Jian R, Tang S, Yin L, Zhou P (2010) RNAi-mediated CD73 suppression induces apoptosis and cell-cycle arrest in human breast cancer cells. Cancer Sci 101(12):2561–2569. 10.1111/j.1349-7006.2010.01733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8(3):437–502. 10.1007/s11302-012-9309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.