Abstract
Purpose
Autophagy, as a highly conserved cellular degradation and recycling process, plays an important part in maintaining cellular homeostasis. PKC signaling is involved in multiple pathways including cell cycle progression, tumorigenesis, migration and autophagy.
Methods
Literatures about PKC and autophagy from PubMed databases were reviewed in this study.
Results
Studies regarding the association of PKC and autophagy remain debatable. Different duration of the stimulation of autophagy and distinct cell contexts result in different function of PKC in regulating autophagy. The subcellular localization of PKCs and their downstream regulators may influence the autophagy regulation as well. As important intracellular components, the mitochondria play an important role in regulating autophagy, by metabolic modulation and structural derangement.
Conclusion
Phase II studies regarding PKC-β inhibitor, enzastaurin, showed promising results in MCL, DLBCL and recurrent high-grade gliomas. However, the detailed mechanism is still in need. The mechanism of PKC-β in mediating autophagy in lymphoma and high-grade gliomas remains elusive as well. Moreover, several studies were in agreement that rottlerin enhanced autophagy in breast cancer cells, which warrants further clinical studies to verify PKC-δ as a therapeutic target. Thus, identifying the function of PKC in modulating autophagy and conducting related clinical studies help find novel target for chemotherapy.
Keywords: Autophagy, PKC-β, PKC-δ, PKC-α, Rottlerin
Introduction
Autophagy is an evolutionarily conserved intracellular degradation and recycling process (Parzych and Klionsky 2014). During the autophagic process, unnecessary or dysfunctional cellular components are degraded and recycled (Klionsky 2008). It could be induced by diverse stressful conditions, including starvation, ionizing radiation and chemotherapeutic agents (Huang et al. 2015; Jiang et al. 2010; Liang et al. 2013). The consequence of autophagy is highly correlated with strength and duration of stimulation and cellular context (Gutierrez et al. 2004; Tanida 2011; Wang and Klionsky 2003). Since autophagy helps maintain the cellular homeostasis and mediate programmed cell death, abnormal autophagic condition was correlated with neurodegenerative diseases, cancer and cardiomyopathy (Shintani and Klionsky 2004). Correspondingly, for anti-tumor therapy, autophagy could deteriorate or ameliorate the cancer treatment efficacy. Multiple signaling pathways are involved in the process of autophagy, including MAPK/JNK, PI3K/AKT/mTOR and protein kinase C (PKC)-related signaling pathway (Choi et al. 2015; Heras-Sandoval et al. 2014; Robert et al. 2009; Yu et al. 2013; Zhang et al. 2009; Zhou et al. 2015). Regulating these pathways, for example, through modulating PKC, will benefit chemotherapy of cancer through autophagic process.
Protein kinase C
As an autophagy regulator, PKC is a group of protein kinase enzymes mediating the function of other proteins through phosphorylating serine and threonine amino acid residues on these proteins. 10 subtypes of PKC have been found in tissue of mammals, which could be divided into three groups: conventional PKC (cPKC), including α, βI, βII and γ subtypes; novel PKC (nPKC), including δ, ε, η and θ subtypes; atypical PKC (aPKC), including ι (also known as λ in mice) and ζ subtypes (Murray et al. 2011). cPKC contains C1 and C2 domains, which could be activated by diacylglycerol (DAG) and Ca2+ in the presence of phosphatidylserine. nPKC, without C2 domain, has to be activated in a DAG- and phospholipid-dependent manner. However, aPKC, for example, PKC-ι, could be stimulated through DAG-, phospholipid- and Ca2+-independent pathway. PKC signaling is involved in multiple pathways through mediating expression of genes relevant for cell cycle progression, tumorigenesis and migration (Garg et al. 2014) (Table 1).
Table 1.
Different isoforms of PKC and related characteristics
| Protein kinase C isoforms | cPKC | nPKC | aPKC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| α | β | γ | δ | ε |
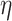
|
θ | ι (also λ) | ζ | |
| Activation | DAG, phosphatidylserine and calcium dependent | DAG and phosphatidylserine dependent | DAG, phosphatidylserine and calcium independent | ||||||
| Activators | PMA | PMA | PMA | PMA | PMA | PMA | PMA | ||
| TPA | TPA | IDB | IDB | IDB | IDB | ||||
| SAG | |||||||||
| Inhibitors | Staurosporine | CGP5335 | Rottlerin | Rottlerin | Rottlerin | ATM | |||
| Ro-31-8220 | LY333531 | Safingol | Safingol | C20 | |||||
| GF109293X | Hispidin | KAI-1678 | |||||||
| Ro-32-0432 | Bis I | ||||||||
| Enzastaurin | |||||||||
The characteristics of different PKC isoforms
PMA phorbol-12-myristate-13-acetate, TPA 12-O-tetradecanoylphorbol-13-acetate, IDB ingenol-3-20-dibenzoate, SAG 1-stearoyl-2-arachidonoylsn-glycerol, Bis I bisindolylmaleimide I, ATM aurothiomalate
PKC regulates autophagy
Among those cellular processes, PKC could help maintain cellular homeostasis through mediating autophagy of cellular components. It was demonstrated that PKC mediated oridonin-induced autophagy in HeLa cells through regulating downstream Raf-1 and JNK signaling. Inhibiting expression of PKC through staurosporine can inhibit autophagy process. In addition, using phorbol 12-myristate 13-acetate (PMA) as a PKC activator can promote autophagy (Zhang et al. 2009). Also, PKC can mediate pseudolaric acid B (PBA) or acadesine-induced autophagy which was confirmed by conversion from LC-3 I to LC-3 II (Robert et al. 2009; Yu et al. 2013). It is noteworthy that the study using acadesine as an autophagy inducer suggested PKC activation required the redistribution into the microsomal fraction from the cytosol (Robert et al. 2009). Hence, these studies indicated that PKC (regardless of different isoforms) had a positive effect on autophagy. However, one study in HEK293 cell demonstrated that PKC inhibited autophagy induced by starvation, confirmed by blockage of autophagosome formation. T6 and T29 of LC3 were phosphorylated by PKC, which was not critical for autophagy (Jiang et al. 2010). Therefore, PKC-mediated autophagy may be in a LC3 phosphorylation-independent process. In contrast, PKA-mediated LC3 phosphorylation at Ser12 dampened the LC3 turnover and redistribution to the phagophore, exhibiting the inhibitory effect of LC3 phosphorylation on autophagy (Xie et al. 2015) (Fig. 1).
Fig. 1.
PKC regulates autophagy. a PKC positively mediates oridonin-induced autophagy in a JNK-dependent manner. b PKC-mediated autophagy is positively mediated by Raf-1. c PKC-induced LC3 phosphorylation cannot regulate autophagy directly. d PKC has to be translocated to microsomal fraction to induce autophagy
cPKC regulates autophagy
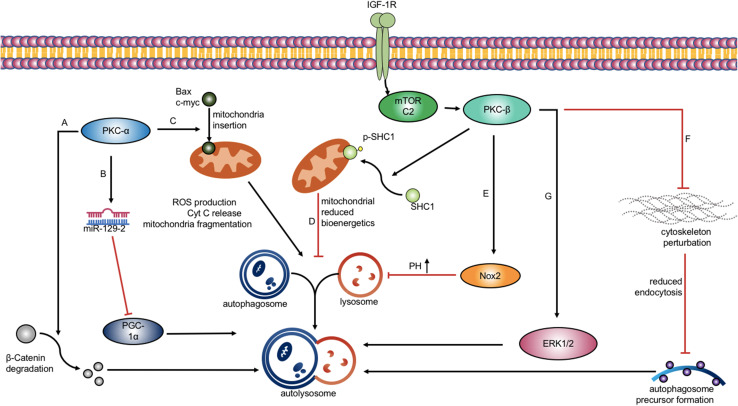
Among cPKC, PKC-α is highly expressed in multiple cancer cells. This common isoform of cPKC promotes the tumorigenesis of several tumors, as a malignant phenotype. More studies consistently demonstrated the positive relationship of PKC-α and autophagy. The IGF-1R depletion inhibited the activity of mTORC2 complex, inducing actin cytoskeleton perturbation in a PKC-α/β-dependent manner. This cytoskeleton derangement resulted in reduced endocytosis and decreased Atg16L1 autophagosome precursors (Renna et al. 2013). β-catenin, as a crucial protein contributing to cytoskeleton formation, helps form the cadherin–catenin complex and acts as an intracellular signal transducer. The β-catenin degradation may be a process paralleled with cytoskeleton perturbation and leads to reduced endocytosis. This β-catenin degradation process could be blocked by inhibiting the expression of PKC-α using Gö6976 and siRNA knockdown. Autophagy induced by glucose deprivation was alleviated as a result (Choi et al. 2015). Moreover, PKC-α could stimulate autophagy through increasing the insertion of Bax c-myc into the mitochondrial membrane, rather than directly phosphorylating Bax c-myc (Silva et al. 2011). The redistribution of Bax c-myc to mitochondria enhanced reactive oxygen species (ROS) production, cyt c release and mitochondrial fragmentation. Simply using PKC inhibitor to decrease phosphorylation may not inhibit the autophagic process, which was in agreement with Jiang et al. (2010). Thus, PKC-α could influence the autophagic process in a phosphorylation-independent manner, but in a mitochondrial-structural-perturbation-dependent way. Interestingly, another study which involved mitochondria modulation revealed that activation of PKC-β resulted in a reduction in mitochondrial bioenergetics, without mitochondrial structural perturbation. This process was paralleled with phosphorylation of SHC1 and its insertion to mitochondrial membrane (Patergnani et al. 2013). The regulation of autophagy was confirmed by decrease of endogenous LC3-II level, increase of SQSTM1/p62 and inhibition of formation of autophagosomes. To be engulfed by an autophagosome, the major axis of a mitochondria has to decrease to 1 µm, compare with its normal value, 5 µm(Patergnani et al. 2013). This pointed out the significance of the fragmentation process or structural modulation of mitochondria in mediating autophagy, which has not been observed by Patergnani et al. (2013). In addition, serum starvation was also implicated to induce the elongation of the mitochondria and maintain the ATP production of mitochondria, and thus, suppressed the autophagic process (Gomes et al. 2011; Rambold et al. 2011). PKC-α tyrosine phosphorylation level was increased when autophagy was stimulated by palmitic acid (PA). This activation of PKC-α may be a result of the accumulation of DAG in cells, directly from PA. Knockdown of PKC-α using siRNA decreased the level of LC3-II and autophagic flux although treated with PA (Tan et al. 2012). On the contrary, one study illustrated a negative effect of PKC-α on autophagy triggered by diabetic pregnancy. PKC-α suppressed expression of PGC-1α through regulating miR-129-2, and thus, inhibited autophagy (Wang et al. 2017). It is noteworthy that the mitochondrial dysfunction was observed as well in this study. But the appearance of LC3-GFP puncta and mitochondrial modulation were induced as early as 6 h after PGC-1α alteration, indicating that the occurrence of autophagy was not a result of mitochondrial biogenesis. PGC-1α could coordinate gene expression relevant for mitochondrial oxidative metabolism and interact with multiple transcriptional factors to induce mitochondrial biogenesis in autophagy-independent way (Puigserver and Spiegelman 2003). Thus, the occurrence of autophagy may not correlate with mitochondrial dysfunction. The contradictory results of studies demonstrated above may be a consequence of a change in the induction of autophagy. Compared with this study which used streptozotocin to induce diabetes-dependent autophagy inhibition, other studies primarily used glucose deprivation, starvation and chemicals for stimulation of autophagy. The PKC-β was reported to suppress autophagy through mediating the palmitate-induced stimulation of Nox2-dervied superoxide, impairment of lysosomal acidification, and then, accumulation of autophagosomes and blockage of autophagic flux (Jaishy et al. 2015). However, an inconsistent study in vivo reported that PKC-βI positively regulated transverse coronary constriction (TAC)-induced cardiac autophagy through phosphorylating ERK1/2 (Weng et al. 2014). The inconsistency may be attributed to the PKC-βI inhibitor, CGP53353, which could also act on PKC-βII and even other nPKCs and aPKCs. PKC-βI knockdown may confirm the result (Fig. 2). Studies on PKC-γ were seldom reported. PKC-γ inhibition may result in elevated autophagy activity through Akt/mTOR reduction to protect neurons from ischemic injury triggered by middle cerebral artery occlusion (MCAO) (Wei et al. 2016).
Fig. 2.
PKC-α/β regulates autophagy through different mechanisms. a PKC-α enhances autophagy by inducing β-Catenin degradation. b PKC-α activates miR-129-2, which negatively regulates PGC-1α and inhibits autophagic process. c PKC-α induces autophagy through mediating Bax c-myc insertion to mitochondria, which leads to ROS production and mitochondria fragmentation. d PKC-β reduces mitochondrial bioenergetics through phosphorylating and inserting SHC1 into mitochondria membrane, and thus, inhibits autophagy. e PKC-β activates Nox2 signaling and then impairs the lysosome acidification process, which results in autophagosome accumulation. f Activated IGF-1R induces mTORC2/ PKC-β signaling pathway, inhibiting cytoskeleton perturbation, which could inhibit autophagy precursor formation. g PKC-β promotes autophagy through ERK1/2 up regulation
nPKC regulates autophagy
As a typical isoform of nPKCs, PKC-δ itself could play a contradictory role in cell survival and cell death through acting as a pro-apoptotic protein during DNA damage-related apoptosis or as an anti-apoptotic protein in cytokine receptor-induced cell death. Studies regarding the association of PKC-δ and autophagy were conflicting as well. The modulation of Akt/mTOR signaling pathway was observed in PKC-δ mediating autophagy. A PKC-δ inhibitor, rottlerin, was demonstrated to induce early autophagy and late apoptosis through phosphorylating AMPK at Thr-172 or inhibiting Akt/mTOR pathway in human breast cancer stem cells (CSCs) (Kumar et al. 2013). Similarly, in rat proximal tubular cell (RPTC) treated with cisplatin, PKC-δ was implicated to activate Akt/mTOR/ULK signaling pathway through directly phosphorylating Akt at Ser473 to suppress autophagy (Zhang et al. 2017). One study in NRK52E kidney cells reported that PKC-δ activated and phosphorylated Serine of GSK3αβ which markedly suppressed the expression of HO-1 and subsequently inhibit autophagy induced by Cd exposure (So and Oh 2016). Interestingly, this study shed light on the influence of different subcellular localization of PKC-δ on autophagy regulation. The translocation of PKC-δ to organelles including the ER and nucleus is correlated with different cell types and stimuli (Zhao et al. 2012). PKC-δ localized in cytosol and mitochondria could play a pro-apoptotic role, while its localization in endoplasmic reticulum (ER) and nucleus has an anti-apoptotic effect (Gomel et al. 2007). As So et al. demonstrated in their study, Cd-activated p-PKC-δ accumulated in nuclear and particulate fractions, suggesting its dual roles in both suppressing autophagy and enhancing apoptosis (So and Oh 2016). Thus, it is reasoned that the autophagy regulation by PKC-δ is dependent on its subcellular localization. One study in pancreatic cancer cells revealed that PKC-δ was negatively associated with autophagy. Using rottlerin as an inhibitor of PKC-δ resulted in a marked increase in autophagy (mediated by downregulation of tissue transglutaminase (TG2)) shown by induction of LC3-II protein and other markers (Akar et al. 2007). Similarly, in lung cancer cells, PKC-δ-mediated CUB domain-containing protein 1 (CDCP1) induced suppression of autophagy. TG2 was reported to be PKC-δ downstream autophagy regulator as well (Uekita et al. 2013). TG2, a calcium-dependent enzyme of the protein-glutamine γ-glutamyltransferases family, performs a crosslinking function between an ε-amino group of a lysine residue and a γ-carboxamide group of glutamine residue of proteins to form a crosslink which is resistant to proteolysis (Facchiano et al. 2006). Interestingly, TG2 has been implicated to play a part in mediating posttranslational modification of proteins during the process of maturation of autophagosomes and helping ubiquitinated proteins recognition by p62 and degradation by autophagolysosomes (D’Eletto et al. 2009, 2012). This function of TG2 indicates its positive role in mediating autophagy. PKC-δ could positively mediate sulfaphenazole (SUL)-induced autophagy in adult rate cardiomyocytes for cardioprotection (Huang et al. 2010). The SUL-induced PKC-δ activation was accompanied by its translocation from the cytosol to a membrane compartment, and the redistribution was highly aligned with α-actinin. α-actinin, as a major component of cytoskeleton, is a substrate of TG2. Thus, it is reasonable to propose that the colocalization of PKC-δ and α-actinin is mediated by TG2. Activated TG2 by PKC-δ stimulates its downstream α-actinin and provides stabilization of the cytoskeleton, which benefits cardioprotection (Almami et al. 2014). The contradictory effect of TG2 on autophagy regulation may be explained by its subcellular distribution and transamidation activity. In addition, several studies had the opposite result, which implicated that PKC-δ was positively associated with autophagy. Shahnazari et al. revealed that PKC-δ induced autophagy in a JNK and NADPH oxidase-dependent pathway. Inhibiting JNK through SP600125 or using siRNA of p22phox (a pivotal component of NOX1-4 NADPH oxidases) showed impairment of anti-bacterial autophagy (Shahnazari et al. 2010). Suppression of PKC-δ was reported to mediate autophagy through STAT/Mcl-1 signaling pathway in mycobacterium tuberculosis-infected macrophages (Kumar et al. 2016). Moreover, hypoxic stress was implicated to induce PKC-δ/JNK1-dependent autophagy. JNK1 relieved the inhibitory effect of Bcl-2 through phosphorylating Bcl-2 and inhibited its interaction with Beclin-1 to promote the autophagic process (Chen et al. 2008). Interestingly, Chen et al. pointed out the lack of specificity of rottlerin as a PKC-δ inhibitor, which also uncouples mitochondria and activates 5′-AMP-activated protein kinase (AMPK) (Chen et al. 2008). Rottlerin could induce autophagy through directly inhibiting mTORC1, in a PKCδ-, ERK-, PI3K/Akt-, Bcl2/Beclin- and AMPK-independent way (Torricelli et al. 2015). But several studies indicated that using rottlerin and siRNA or dominant negative mutant PKC-δ to inhibit PKC-δ had the same result (Berardi et al. 2016; Kumar et al. 2016; Shahnazari et al. 2010). According to one study using RGC5 cells, PKC-δ played a dual role on regulating ethambutol (EMB)-induced autophagy. Initially, PKC-δ activated by EMB inhibited PI3k/Akt/mTOR/p70S6K signaling to promote autophagy. As the EMB stimulation prolonged, autophagosomes accumulated and autophagy flux was impaired. But rottlerin attenuated autophagosome accumulation and restored autophagy flux (Huang et al. 2015) (Fig. 3). Thus, stimulating autophagy through different way in various cells and tissues and alteration of duration of the stimulation may lead to distinct result. Not only PKC-δ, PKC-ε was demonstrated to regulate autophagy. It was pointed out that autophagy could be triggered with inhibition of PKC-ε, to regulate mTOR/p70S6K and MAPK (ERK) pathway (Coward et al. 2009). However, one study in Hela cells found a positive association of PKC-ε and autophagy. Using zapotin to down-modulate PKC-ε resulted in inhibition of autophagosome formation and decline in LC3 protein levels (Toton et al. 2016). mTOR was also activated by zapotin treatment. Another nPKC, PKC-θ-mediated hypoxia induced autophagy in rat hepatic stellate cells (HSCs) through Ca2+-dependent pathway (Jin et al. 2016). Although Ca2+–AMPK–mTOR pathway was found to mediate hypoxic stress induced autophagy parallel to Ca2+–PKCθ pathway, this study did not investigate the interaction between this two pathways. It was reported that PKC-θ activated p38-MAPK signaling and mediated autophagy (induced by TPA with sodium butyrate) in EBV-infected B cells (Gonnella et al. 2015). Interestingly, they also revealed that PKC-θ dephosphorylated Akt to balance the cell survival and cell death. In addition, PKC-θ was implicated to mediate endoplasmic reticulum stress (ER stress)-induced autophagy, independent with JNK (Madaro et al. 2013). A similar result was reported that PKC-θ regulated ER stress (stimulated by thapsigargin) induced autophagy. PKC-θ was activated by Ca2+ and phospholipase C-dependent pathway. And the activation of PKC-θ at Thr538 and its membrane localization may enhance the dynamic membrane changes to regulate autophagic process (Sakaki and Kaufman 2008; Sakaki et al. 2008). When investigating the role of nPKC in a certain kind of organ, it is promising to see the consistent results regarding breast cancer (Berardi et al. 2016; Coward et al. 2009; Kumar et al. 2013). These studies suggested the inhibiting effect of PKC-δ and PKC-ε on autophagy, indicating inhibitors of these two isoforms of nPKC could benefit cancer therapeutics.
Fig. 3.
PKC-δ regulates autophagy through different mechanisms. a PKC-δ up regulates JNK expression, which releases Beclin-1 from Bcl2/Beclin-1 complex and promotes autophagy. b PKC-δ up regulates STAT and induces the combination of Mcl and Beclin-1, which inhibits the autophagy. c PKC-δ activates TG2, which helps ubiquitinated protein recognition by p62 and autophagosome maturation. d PKC-δ inhibits autophagy through directly phosphorylating Akt and activating Akt/mTOR/ULK signaling pathway. e PKC-δ stimulates autophagy through inhibiting PI3K/Akt/mTOR/ULK pathway. f PKC-δ induces autophagy in a NADPH oxidase-dependent pathway. g PKC-δ inhibits autophagy through directly phosphorylating and activating GSK3αβ, which inhibits downstream HO-1
aPKC regulates autophagy
Generally, aPKC was indicated to mediate autophagy negatively in Drosophila as opposed to Kibra which was confirmed by mCherry-Atg8a spots. It was pointed out that PKC-ι was negatively correlated with autophagy through inhibiting formation of autophagosome and degradation of autophagic substrates. PRKCI depletion enhanced autophagy by inducing inactivation of PIK3CA/Akt-mTOR pathway (Qu et al. 2016). In agreement with this study, it was reported that PKC-ι suppressed autophagy through inhibiting degradation of autophagic substrates, including heat shock cognate protein 70 (Hsc70) and β-catenin (Wang et al. 2013, 2014).
Summary
Autophagy as an important cellular process which regulates the degradation of cellular organelles and cellular homeostasis has been implicated in various aspects of human health and pathophysiology. As a crucial autophagic regulator, PKC appears to be a potential target for clinical therapeutics. Multiple inhibitors of PKC have been used in clinical trials to verify PKC as a potential target for cancer therapy. In a phase II study in 60 patients with relapsed or refractory mantle cell lymphoma (MCL), 22 patients were free from progression (FFP) for ≥ 3 cycles (one cycle = 28 days) and 6 patients of those were FFP for ≥ 6 months with the treatment of enzastaurin, a PKC-β and PI3K/Akt pathway inhibitor (Morschhauser et al. 2008). Enzastaurin was also implicated as an effective agent for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and recurrent high-grade gliomas in phase II studies (Fine et al. 2005; Robertson et al. 2007). Aurothiomalate (ATM), a PKC-ι inhibitor, efficiently suppressed the cellular transformation of non-small cell lung cancer (NSCLC) through directly targeting Cys69 within the PB1 domain of PKC-ι. Related Phase I clinical trial of ATM is ongoing (Fields and Murray 2008). Therefore, PKC, especially PKC-β and PKC-ι, provides potential target for increasing cancer therapeutic efficacy. However, studies seldom report the role of enzastaurin, as a PKC-β inhibitor, in modulating autophagy and whether PKC-β also plays a part in regulating autophagy in lymphoma. More studies regarding its mechanism are needed. Based on the studies mentioned in this review, different isoforms of PKC had inconsistent influence on autophagy. Distinct duration of the stimulation of autophagy and induction of PKC could be the reason. PKC-δ plays “dual roles” in both cell survival and death. Early stimulation of PKC-δ may lead to an adaptive and protective response through activating autophagy, but prolonged activation of PKC-δ could contribute to cell death (Chen et al. 2008). Moreover, different duration of the stimulation or induction of autophagy leads to distinct outcomes (Huang et al. 2015). PKC-δ is implicated to promote autophagy as a result of relatively transient stimulation through increasing the formation of autophagosomes and degradation of autophagic substrates. As the stimulation prolongs, PKCδ inhibits autophagy which is indicated by accumulation of autophagosomes and blockage of autophagic flux. However, the detailed mechanism remains unknown. In addition, as was suggested by a study of autophagy triggered by acute hypoxic stress, rottlerin was not a PKCδ-specific inhibitor, which could also uncouple mitochondria and activates AMPK signaling pathway (Chen et al. 2008). However, in several studies using rottlerin as PKC-δ inhibitor, siRNA was used for PKC-δ inhibition as well. From the perspective of breast cancer, rottlerin, the inhibitor of PKC-δ, was demonstrated to enhance the process of autophagy consistently based on three studies, which implicates its role as a therapeutic target. More clinical trials are in need to verify its role for breast cancer treatment. In addition, it is interesting to mention the influence of subcellular localization of PKC and its downstream molecules (such as TG2) on autophagy regulation. Cd-activated p-PKC-δ distributed in nuclear and particulate fractions, suggesting its dual function in regulating both autophagy and apoptosis (So and Oh 2016). Different types of cells may exhibit different intracellular distribution of PKC, while different stimuli may induce its translocation to different organelles. Hence, this could be one explanation of these contradictory results.
As an important intracellular organelle, mitochondria play an essential role in autophagy regulation. On the one hand, autophagy counteracts the accumulation of ROS induced by mitochondrial dysfunction and regulates the cellular metabolic bioenergetics (Hill et al. 2012; Liesa and Shirihai 2013). On the other hand, dysfunctional mitochondria could be selectively eliminated through mitophagy in a physiological condition, to maintain the cellular homeostasis (Al Rawi et al. 2011; Sandoval et al. 2008). It was mentioned in this review that PKC-α could positively regulate autophagy through inducing mitochondrial structural perturbation, ROS accumulation and Cyt C release (Silva et al. 2011); while PKC-β induced a reduction of mitochondria metabolic bioenergetics, without the structural modulation, and exhibited a lower autophagy activity (Patergnani et al. 2013). Moreover, although mitochondrial modulation was observed when autophagy was inhibited by PKC-α/miR-129-2/PGC-1α axis, its occurrence was as early as the autophagy inhibition. Thus, it is reasonable to think that the structural modulation of mitochondria positively induces autophagy through accumulation of ROS, while the reduced metabolic bioenergetics has a negative effect on autophagy. PKC-θ enhanced autophagy consistently according to these studies, while PKC-ι negatively regulates autophagy. But more studies are still needed to verify their function in autophagy regulation. Considering the conflicting results, further studies about mechanisms of autophagy regulated by PKC are needed to identify its role during the autophagic process. Identifying the function of PKC on autophagy and the detailed mechanism helps find novel target for chemotherapy. Autophagy could be enhanced or inhibited through inducing or dampening PKC and its downstream signaling molecules. Regulating the death of cancer cell or homeostasis of normal cell through these pathways may offer great possibilities for clinical treatment.
Acknowledgements
We are grateful to the staff at the NHC Key Laboratory of Radiobiology (Ministry of Health).
Funding
This study was supported by National Natural Science Foundation of China (NSFC) grants [grant number 31500682].
Compliance with ethical standards
Conflict of interest
Tianyi Wang declares that he has no conflict of interest. Conghe Liu declares that she has no conflict of interest. Lili Jia declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G (2007) Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res 5:241–249. 10.1158/1541-7786.MCR-06-0229 [DOI] [PubMed] [Google Scholar]
- Al Rawi S et al (2011) Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 334:1144–1147. 10.1126/science.1211878 [DOI] [PubMed] [Google Scholar]
- Almami I, Dickenson JM, Hargreaves AJ, Bonner PL (2014) Modulation of transglutaminase 2 activity in H9c2 cells by PKC and PKA signalling: a role for transglutaminase 2 in cytoprotection. Br J Pharmacol 171:3946–3960. 10.1111/bph.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi DE et al (2016) PKCdelta inhibition impairs mammary cancer proliferative capacity but selects cancer stem cells involving autophagy. J Cell Biochem 117:730–740. 10.1002/jcb.25358 [DOI] [PubMed] [Google Scholar]
- Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK (2008) Novel roles for protein kinase Cdelta-dependent signaling pathways in acute hypoxic stress-induced autophagy. J Biol Chem 283:34432–34444. 10.1074/jbc.M804239200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Song JK, Yim YS, Yun HG, Chun KH (2015) Glucose deprivation triggers protein kinase C-dependent beta-catenin proteasomal degradation. J Biol Chem 290:9863–9873. 10.1074/jbc.M114.606756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward J et al (2009) Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-. kinase pathway. Autophagy 5:184–193 [DOI] [PubMed] [Google Scholar]
- D’Eletto M et al (2009) Transglutaminase 2 is involved in autophagosome maturation. Autophagy 5:1145–1154 [DOI] [PubMed] [Google Scholar]
- D’Eletto M et al (2012) Type 2 transglutaminase is involved in the autophagy-dependent clearance of ubiquitinated proteins. Cell Death Differ 19:1228–1238. 10.1038/cdd.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchiano F, Facchiano A, Facchiano AM (2006) The role of transglutaminase-2 and its substrates in human diseases. Front Biosci 11:1758–1773 [DOI] [PubMed] [Google Scholar]
- Fields AP, Murray NR (2008) Protein kinase C isozymes as therapeutic targets for treatment of human cancers. Adv Enzyme Regul 48:166–178. 10.1016/j.advenzreg.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine HA et al (2005) Results from phase II trial of enzastaurin (LY317615) in patients with recurrent high grade gliomas. J Clin Oncol 23:115S [Google Scholar]
- Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG (2014) Protein kinase C and cancer: what we know and what we do not. Oncogene 33:5225–5237. 10.1038/onc.2013.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomel R, Xiang C, Finniss S, Lee HK, Lu W, Okhrimenko H, Brodie C (2007) The localization of protein kinase Cdelta in different subcellular sites affects its proapoptotic and antiapoptotic functions and the activation of distinct downstream signaling pathways. Mol Cancer Res 5:627–639. 10.1158/1541-7786.MCR-06-0255 [DOI] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13:589–598. 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnella R, Granato M, Farina A, Santarelli R, Faggioni A, Cirone M (2015) PKC theta and p38 MAPK activate the EBV lytic cycle through autophagy induction. Biochim Biophys Acta 1853:1586–1595. 10.1016/j.bbamcr.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753–766. 10.1016/j.cell.2004.11.038 [DOI] [PubMed] [Google Scholar]
- Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701. 10.1016/j.cellsig.2014.08.019 [DOI] [PubMed] [Google Scholar]
- Hill BG, Benavides GA, Lancaster JR Jr, Ballinger S, Dell’Italia L, Jianhua Z, Darley-Usmar VM (2012) Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem 393:1485–1512. 10.1515/hsz-2012-0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C et al (2010) Autophagy and protein kinase C are required for cardioprotection by sulfaphenazole. Am J Physiol Heart Circ Physiol 298:H570–H579. 10.1152/ajpheart.00716.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SP, Chien JY, Tsai RK (2015) Ethambutol induces impaired autophagic flux and apoptosis in the rat retina. Dis Model Mech 8:977–987. 10.1242/dmm.019737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaishy B et al (2015) Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. J Lipid Res 56:546–561. 10.1194/jlr.M055152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Cheng D, Liu W, Peng J, Feng J (2010) Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem Biophys Res Commun 395:471–476. 10.1016/j.bbrc.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Bai Y, Ni H, Qiang L, Ye L, Shan Y, Zhou M (2016) Activation of autophagy through calcium-dependent AMPK/mTOR and PKCtheta pathway causes activation of rat hepatic stellate cells under hypoxic stress. FEBS Lett 590:672–682. 10.1002/1873-3468.12090 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ (2008) Autophagy revisited: a conversation with Christian de Duve. Autophagy 4:740–743 [DOI] [PubMed] [Google Scholar]
- Kumar D, Shankar S, Srivastava RK (2013) Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer 12:171. 10.1186/1476-4598-12-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R et al (2016) MicroRNA 17-5p regulates autophagy in Mycobacterium tuberculosis-infected macrophages by targeting Mcl-1 and STAT3. Cell Microbiol 18:679–691. 10.1111/cmi.12540 [DOI] [PubMed] [Google Scholar]
- Liang N et al (2013) ATM pathway is essential for ionizing radiation-induced autophagy. Cell Signal 25:2530–2539. 10.1016/j.cellsig.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17:491–506. 10.1016/j.cmet.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaro L, Marrocco V, Carnio S, Sandri M, Bouche M (2013) Intracellular signaling in ER stress-induced autophagy in skeletal muscle cells. FASEB J 27:1990–2000. 10.1096/fj.12-215475 [DOI] [PubMed] [Google Scholar]
- Morschhauser F et al (2008) A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Ann Oncol 19:247–253. 10.1093/annonc/mdm463 [DOI] [PubMed] [Google Scholar]
- Murray NR, Kalari KR, Fields AP (2011) Protein kinase Ciota expression and oncogenic signaling mechanisms in cancer. J Cell Physiol 226:879–887. 10.1002/jcp.22463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20:460–473. 10.1089/ars.2013.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patergnani S, Marchi S, Rimessi A, Bonora M, Giorgi C, Mehta KD, Pinton P (2013) PRKCB/protein kinase C, beta and the mitochondrial axis as key regulators of autophagy. Autophagy 9:1367–1385. 10.4161/auto.25239 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90. 10.1210/er.2002-0012 [DOI] [PubMed] [Google Scholar]
- Qu L, Li G, Xia D, Hongdu B, Xu C, Lin X, Chen Y (2016) PRKCI negatively regulates autophagy via PIK3CA/AKT-MTOR signaling. Biochem Biophys Res Commun 470:306–312. 10.1016/j.bbrc.2016.01.059 [DOI] [PubMed] [Google Scholar]
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 108:10190–10195. 10.1073/pnas.1107402108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M et al (2013) IGF-1 receptor antagonism inhibits autophagy. Hum Mol Genet 22:4528–4544. 10.1093/hmg/ddt300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert G et al (2009) Acadesine kills chronic myelogenous leukemia (CML) cells through PKC-dependent induction of autophagic cell death. PLoS One 4:e7889. 10.1371/journal.pone.0007889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MJ et al (2007) Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol 25:1741–1746. 10.1200/JCO.2006.09.3146 [DOI] [PubMed] [Google Scholar]
- Sakaki K, Kaufman RJ (2008) Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy 4:841–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki K, Wu J, Kaufman RJ (2008) Protein kinase Ctheta is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem 283:15370–15380. 10.1074/jbc.M710209200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J (2008) Essential role for Nix in autophagic maturation of erythroid cells. Nature 454:232–235. 10.1038/nature07006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnazari S et al (2010) A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy. Cell Host Microb 8:137–146. 10.1016/j.chom.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306:990–995. 10.1126/science.1099993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RD, Manon S, Goncalves J, Saraiva L, Corte-Real M (2011) Modulation of Bax mitochondrial insertion and induced cell death in yeast by mammalian protein kinase. Calpha Exp Cell Res 317:781–790. 10.1016/j.yexcr.2010.12.001 [DOI] [PubMed] [Google Scholar]
- So KY, Oh SH (2016) Cadmium-induced heme-oxygenase-1 expression plays dual roles in autophagy and apoptosis and is regulated by both PKC-delta and PKB/Akt activation in NRK52E kidney cells. Toxicology 370:49–59. 10.1016/j.tox.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Tan SH, Shui G, Zhou J, Li JJ, Bay BH, Wenk MR, Shen HM (2012) Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin. J Biol Chem 287:14364–14376. 10.1074/jbc.M111.294157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I (2011) Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal 14:2201–2214. 10.1089/ars.2010.3482 [DOI] [PubMed] [Google Scholar]
- Torricelli C et al (2015) Phosphorylation-independent mTORC1 inhibition by the autophagy inducer. Rottlerin Cancer Lett 360:17–27. 10.1016/j.canlet.2015.01.040 [DOI] [PubMed] [Google Scholar]
- Toton E, Romaniuk A, Budzianowski J, Hofmann J, Rybczynska M (2016) Zapotin (5,6,2′,6′-tetramethoxyflavone) modulates the crosstalk between autophagy and apoptosis pathways in cancer cells with overexpressed constitutively active. PKC Nutr Cancer 68:290–304. 10.1080/01635581.2016.1134595 [DOI] [PubMed] [Google Scholar]
- Uekita T, Fujii S, Miyazawa Y, Hashiguchi A, Abe H, Sakamoto M, Sakai R (2013) Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells. Cancer Sci 104:865–870. 10.1111/cas.12154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CW, Klionsky DJ (2003) The molecular mechanism of autophagy. Mol Med 9:65–76 [PMC free article] [PubMed] [Google Scholar]
- Wang BS et al (2013) PKCiota counteracts oxidative stress by regulating Hsc70 in an esophageal cancer cell line. Cell Stress Chaperones 18:359–366. 10.1007/s12192-012-0389-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS et al (2014) Inhibition of atypical protein kinase Ciota induces apoptosis through autophagic degradation of beta-catenin in esophageal cancer cells. Mol Carcinog 53:514–525. 10.1002/mc.22003 [DOI] [PubMed] [Google Scholar]
- Wang F et al (2017) Protein kinase C-alpha suppresses autophagy and induces neural tube defects via miR-129-2 in diabetic pregnancy. Nat Commun 8:15182. 10.1038/ncomms15182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H et al (2016) cPKCgamma-modulated autophagy in neurons alleviates ischemic injury in brain of mice with ischemic stroke through Akt-mTOR. Pathw Transl Stroke Res 7:497–511. 10.1007/s12975-016-0484-4 [DOI] [PubMed] [Google Scholar]
- Weng LQ et al (2014) Aliskiren ameliorates pressure overload-induced heart hypertrophy and fibrosis in mice. Acta Pharmacol Sin 35:1005–1014. 10.1038/aps.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Kang R, Sun X, Zhong M, Huang J, Klionsky DJ, Tang D (2015) Posttranslational modification of autophagy-related proteins. in macroautophagy. Autophagy 11:28–45. 10.4161/15548627.2014.984267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J et al (2013) Pseudolaric acid B induced cell cycle arrest, autophagy and senescence in murine fibrosarcoma l929 cell. Int J Med Sci 10:707–718. 10.7150/ijms.5726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu Y, Tashiro S, Onodera S, Ikejima T (2009) Involvement of PKC signal pathways in oridonin-induced autophagy in HeLa cells: a protective mechanism against apoptosis. Biochem Biophys Res Commun 378:273–278. 10.1016/j.bbrc.2008.11.038 [DOI] [PubMed] [Google Scholar]
- Zhang D, Xu X, Dong Z (2017) PRKCD/PKCdelta contributes to nephrotoxicity during cisplatin chemotherapy by suppressing autophagy. Autophagy 13:631–632. 10.1080/15548627.2016.1269990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Xia L, Chen GQ (2012) Protein kinase cdelta in apoptosis: a brief overview. Arch Immunol Ther Exp (Warsz) 60:361–372. 10.1007/s00005-012-0188-8 [DOI] [PubMed] [Google Scholar]
- Zhou YY, Li Y, Jiang WQ, Zhou LF (2015) MAPK/JNK signalling: a potential autophagy regulation pathway Biosci Rep. 10.1042/BSR20140141 [DOI] [PMC free article] [PubMed] [Google Scholar]