Abstract
Purpose
To validate a previously characterized 10-gene signature in prostate cancer with implication to distinguish aggressive and indolent disease within low and intermediate patients’ risk groups.
Methods
A case–control study design used to select 545 patients from the Mayo clinic tumor registry who underwent radical prostatectomy. A training set from this cohort (n = 359) was used to build a 10-gene model, based on high-dimensional discriminant analysis (HDDA10) to predict several endpoints of clinical patients’ outcome. An independent set (n = 219) from the same institution was used as validation set.
Results
HDDA10 showed significant performance for predicting metastasis (Mets) (AUC 0.68, p = 6.4E – 6) and biochemical recurrence (BCR) (AUC = 0.65, p = 0.003) in the validation set outperforming Gleason grade grouping (GG) for BCR (AUC 0.57, p = 0.03) and with comparable performance for Mets endpoint (GG AUC 0.66, p = 8.1E – 5). HDDA10 prognostic significance was superior to any clinical–pathological parameter within GG2 + 3 (GS7) patients achieving an AUC of 0.74 (p = 0.0037) for BCR compared to Gleason pattern 4 (AUC 0.64) (p = 0.015) and AUC for Mets of 0.68 versus AUC of 0.65 for Gleason pattern 4 (p = 0.01). HDDA10 remained significant for both BCR and Mets in multivariate analysis, suggesting that it can be used to increase accuracy in stratifying patients eligible for active surveillance.
Conclusion
HDDA10 is of added value to GG and other clinical–pathological parameters in predicting BCR and Mets endpoint, especially in the low to intermediate patients’ risk groups. HDDA10 prognostic value should be further validated prospectively in stratifying patients specifically in low to intermediate GS (GG1-2), such as active surveillance programs.
Electronic supplementary material
The online version of this article (10.1007/s00432-018-2615-7) contains supplementary material, which is available to authorized users.
Keywords: Genetic classifier, Grade grouping, Gleason score, Biomarkers, Biochemical recurrence, Clinical metastasis, Prostate cancer, Prognosis
Introduction
Prostate cancer (PCa) is considered one of the most heterogeneous tumors and one that has many genetic alterations associated with its progression pathways (Cazares et al. 2010; Goldenberg et al. 2011; Taylor et al. 2010). Current clinical and pathological parameters as well as clinical nomograms such as D’Amico and Kattan are used in daily practices to aid physicians in their decisions to offer the best available treatment plans related to surgical or radiotherapy interventions (Carter and Isaacs 2004). However, those clinical nomograms do not fare well in predicting outcome for low- and intermediate-risk patients likely to be candidates for active surveillance (Iremashvili et al. 2013). To date, not all patients with Grade Group 1 (Gleason score 6) and GG2 (GS3 + 4) are offered active surveillance due to the likelihood of harboring higher GG in their prostatectomy that may have been under-sampled or misinterpreted in the needle biopsy material due to tumor extent or location in the tissue core. It is projected that such upgrade in GG may reach 30% of all patients with GG1 and 2 (Epstein et al. 2012). Moreover, it is estimated that 10–15% of patients under active surveillance will experience disease progression on yearly basis (Bul et al. 2012; Odom et al. 2014) and, while high cure rates have been observed for patients with GG1 & 2 after radical prostatectomy (82–94%), this decreases significantly for patients with predominant pattern 4 (Pierorazio et al. 2013). Moreover, radical prostatectomies after active surveillances do not always show insignificant disease (Sundi et al. 2013). Currently, clinicians cannot project accurately how often patients will show disease progression if left untreated, as the underlying molecular events responsible for this are not fully characterized.
Multiple single biomarkers and gene panels have been suggested to predict clinical outcome (Andren et al. 2007; Attard et al. 2008; Barros-Silva et al. 2011; Bismar et al. 2006, 2012; Darnel et al. 2009; Demichelis et al. 2007; Sheikh et al. 2008; Fall et al. 2007; Halvorsen et al. 2003; Kibel et al. 2007; Li et al. 2011; Reid et al. 2010). However, to date, these have not been able to make their way into clinical practice, either because they do not provide significant improvement over clinical–pathological parameters (Mucci et al. 2008) or because additional studies fail to replicate their prognostic value.
Our group has recently characterized a 10-gene molecular signature for biochemical recurrence and lethal disease (Bismar et al. 2013). The signature was derived based on differential expression relative to the presence or absence of ERG gene rearrangements, which has been characterized as being the most common genetic rearrangement associated with PCa (Tomlins et al. 2005).
In our initial study, the 10-gene signature showed prognostic significance in three independent public cohorts (Swedish GSE8402, Taylor GSE21032 and Glinsky) and was of added prognostic significance in a case–control study within the Physician Health study (Sboner et al. 2010; Glinsky et al. 2004; Taylor et al. 2010). Furthermore, the 10-gene signature was still prognostic in multivariate model implementing needle biopsy GS and pre-biopsy serum PSA (Taylor cohort).
In this study, we further refine and validate our previously Characterized 10-gene signature using a high-dimensional discriminant analysis (HDDA) to reclassify reliably the 10-gene model (HDDA10) using the Mayo clinic cohort in the prediction of biochemical recurrence and clinical metastasis. This new classification is amenable to individual patients and could be reliably assessed within a well-defined protocol using the Affymetrix Human Exon 1.0 ST GeneChips array.
Materials and methods
Patient cohorts and samples
Patients in this study were selected from the Mayo Clinic Radical Prostatectomy Tumor Registry. Patients received radical prostatectomy for primary prostatic adenocarcinoma as first-line treatments. The study utilized two groups of patients to validate the prognostic significance of the HDDA10 gene signature. The training set (n = 359) was selected using a nested case–control (Erho et al. 2013) and the independent validation set (n = 219) (Karnes et al. 2013) was selected using a case cohort design. We also used several radical prostatectomy and TURP samples from the University of Calgary-Rokyview General Hospital to investigate HDDA10 score relative to multiple tumor foci within individual patient’s tumor and in indolent, advanced and castrate-resistant disease. The study was approved by the institutional review boards at the University of Calgary, Calgary, Alberta and the Mayo Clinic, Rochester, Minnesota.
Expression profiling
RNA was extracted and hybridized to Human Exon 1.0 ST GeneChips following manufacturer’s recommendations as described (Erho et al. 2013; Karnes et al. 2013). Expression data used in this study were obtained from the same two studies. In this study, the training set (n = 359) was obtained from Erho et al. to build the model and validate it using the validation set from Karnes 2013 (n = 219). The Human Exon array expression data corresponding to this study are available from the NCBI’s Gene Expression Omnibus database (GSE46691, GSE62116). The expression data were normalized using SCAN normalization method and data were summarized at core level using fRMA R package (Piccolo et al. 2012).
High-dimensional discriminant analysis (HDDA) 10-gene signature model
A high-dimensional discriminant analysis (HDDA) machine-learning algorithm (Bergé et al. 2012) which is available as R package HDclassif was used to build a classifier using the 10 genes. HDDA estimates model parameters using the maximum likelihood method. The 359 training samples from Erho et al. (2013) were used to estimate the weight of each gene (Erho et al. 2013). The model was trained to predict the metastasis endpoint and then applied to predict the metastasis and BCR endpoints in the 219 samples in Karnes et al. (2013), which were from independent case–cohort. The HDDA model generates probabilistic values between 0 and 1; the larger the value, the higher the risk. The 10 genes have different weights; FRZB, LEF1, SDCBP, WNT2, ING3, and ANK3 have positive weights, while MEIS2, ANXA4, PLA2G7 and CHD5 have negative weight. MEIS2 and SDCBP are the top two genes contributing to the model. We used a cut-off of 0.5 to define high- and low-HDDA10; patients with score greater than 0.5 are annotated as high HDDA10.
Statistical analysis
Discrimination power of the proposed model was measured by area under the receiver operating characteristic curve (AUC). Univariate (UVA) and multivariate (MVA) Cox proportional hazards models for case cohorts were used for risk ratio estimation including clinical and pathological parameters. Survival analysis was conducted using Kaplan–Meier curves to estimate the probability of event-free survival. All statistical analyses were conducted in R 3.0 Statistical software.
Results
The mayo clinic study population demographics
This cohort represented a high-risk patients’ population who underwent radical prostatectomy as initial therapy. In the training set, 72% experienced BCR and 40% showed metastasis. The median time to BCR and metastasis was 3.4 and 5.4 years, respectively. In the testing set, 53% experienced BCR and 32% showed metastasis. The median time to BCR and metastasis was 4 and 5.6 years, respectively. Supp Table 1 demonstrates the clinical and pathological features of the combined cohort.
Comparison of the HDDA10 molecular gene classifier to Gleason score and other parameters in predicting patient’s biochemical recurrence and clinical metastasis
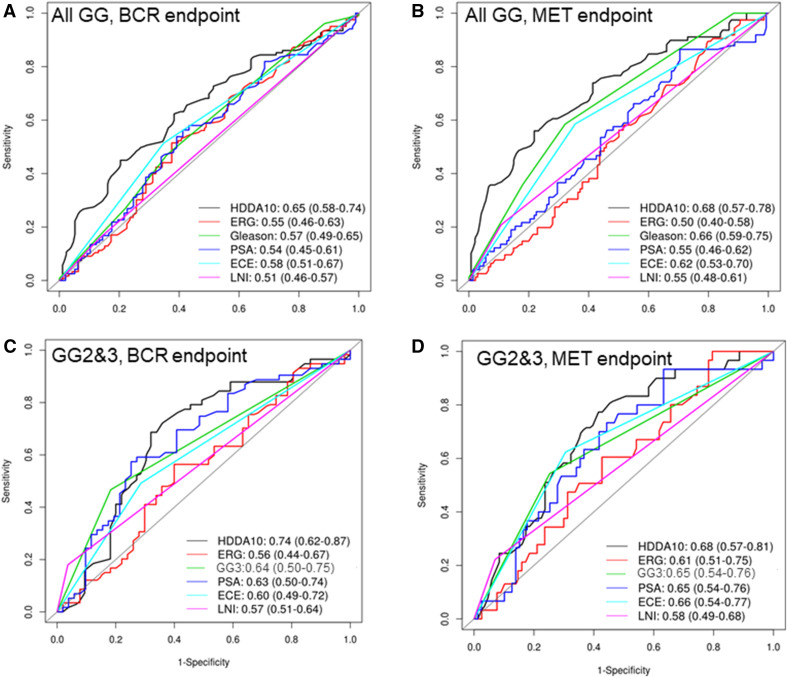
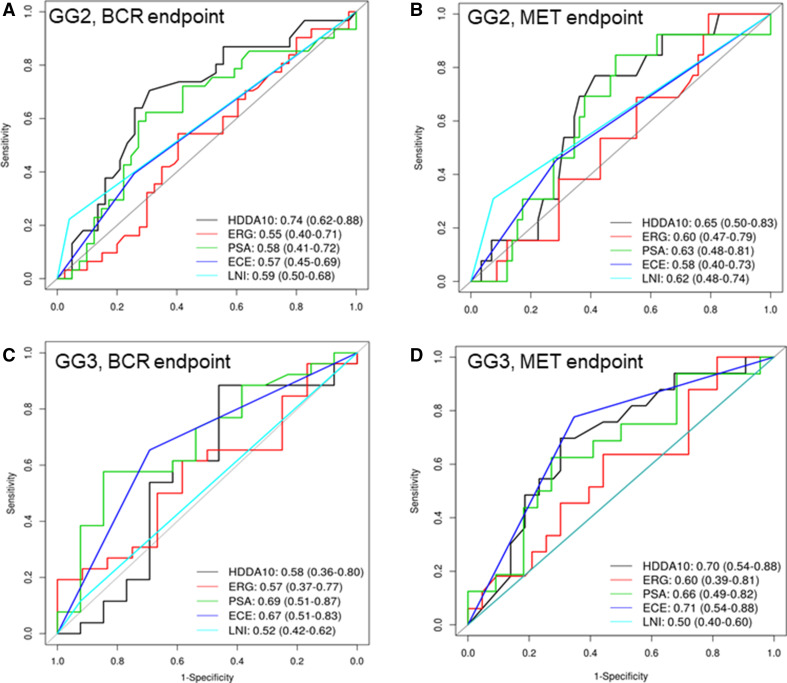
The HDDA10 gene classifier showed significant performance in predicting metastasis (Mets) (AUC 0.68, p = 6.4E–6) and biochemical recurrence (BCR) (AUC = 0.65, p = 0.003) in the validation set, outperforming GG for BCR (GS AUC 0.57, p = 0.03) and with comparable performance to GG for the Mets endpoint (GG AUC 0.66, p = 8.1E–5) (Fig. 1a, b). Limiting the analysis to the subgroup of patients with GG2 & 3, since those are the two groups most likely to benefit from AS options, HDDA10 was most significant in predicting BCR achieving an AUC of 0.74 (p = 0.0037) for BCR compared to Gleason pattern 4 (AUC 0.64) (p = 0.015). The HDDA10 AUC for Mets was slightly lower compared to BCR but still higher to that observed in Gleason pattern 4 (GG3) (AUC 0.68 vs. 0.65) (p = 0.01) (Fig. 1c, d). Other clinical and pathological parameters including ERG status showed comparable performance for BCR and Mets, but were still below those observed for HDDA10. HDDA10 was more informative compared to any other pathological or clinical parameters in patients with GG2 and showed similar values in GG3 suggesting that HDDA10 can be potentially used to predict patients within GG2–3 better than clinical utilization of GG2 versus GG3 (Fig. 2a–d).
Fig. 1.
Survival AUC curves to assess the HDDA10 classifier and other clinico-patholgical variables to predict biochemical recurrence (BCR) and metastasis (Mets) post radical prostatectomy. a Survival AUC to predict BCR in all the samples. b Survival AUC to predict Mets in all samples. c Survival AUC to predict BCR in GG2–3 samples. d Survival AUC to predict Mets in GG2–3 samples. HDDA10 had the highest AUC compared to the single clinico-pathological variables in all samples and the GG2–3 subsets to predict both endpoints
Fig. 2.
Survival AUC curves to assess the HDDA10 classifier and other clinico-patholgical variables to predict biochemical recurrence (BCR) and metastasis (Mets) post radical prostatectomy. a Survival AUC to predict BCR in GG2. b Survival AUC to predict Met in GG2. c Survival AUC to predict BCR in GG3. d Survival AUC to predict Mets in GG3. HDDA10 had the highest AUC compared to the single clinico-pathological variables in the GG2 and PSA had the highest AUC in the GG3 subset
Association of HDDA10 to biochemical recurrence and clinical metastasis in Kaplan–Meier analysis
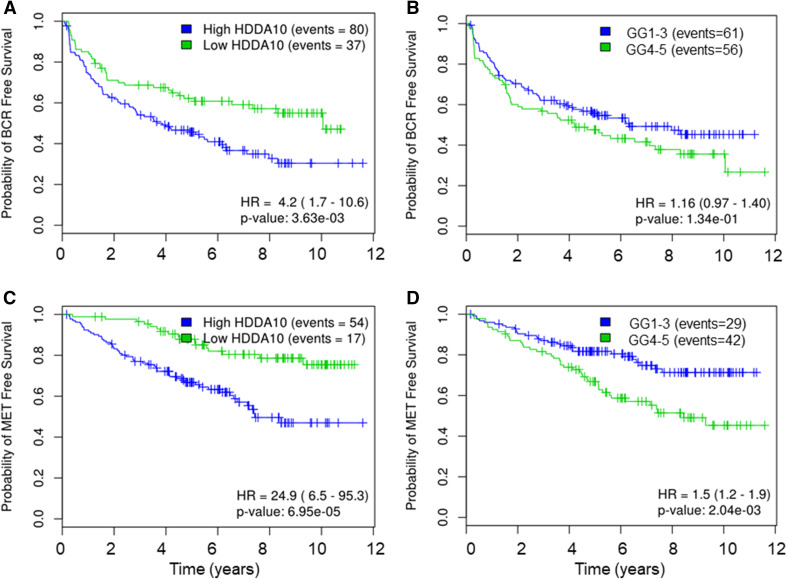
Herein, we compared the prognostic significance of HDDA10 against GG grouped into (GG1-3 vs. GG4-5) in relation to time to BCR and clinical metastasis. HDDA10 was significantly associated with BCR (p = 0.003) (HR 4.2; 95% CI 1.7–10.6) and Mets (p = 6.95e-05) (HR 24; 95% CI 6.5–95.3) which was more significant compared to GG1-3 versus GG4-5 (BCR: p = 0.13, HR 1.16; CI 0.97–1.4, Mets: p = 0.002, HR 1.5 CI 1.2–1.9) (Fig. 3). Here, we used GG1–3 versus GG4–5 grouping as we needed a fair comparison with HDDA10 that splits patients into two groups. As our aim was to investigate HDDA10 prognostic value in low/intermediate GG and as it is well documented that GG2 versus GG3 have an impact on clinical decision specifically for active surveillance programs, we compared our signature model to the two groups of GS7 (3 + 4 (GG2) vs. 4 + 3 (GG3)). HDDA10 maintained superior prognostic value compared to GG within the group of patients with GG2 versus GG3 at both BCR (p = 9.05e-4, HR 8.3; CI 2–34.2) and Mets, (p = 0.0024, HR 41.6 CI 4.3–400) (Fig. 4), again supporting that HDDA10 can be potentially used to predict patients within this subgroup of patients likely eligible to be managed by active surveillance.
Fig. 3.
Kaplan–Meier curves of the HDDA10 and pathological Gleason group in all samples to predict time to BCR and Mets. KM curves of HDDA10 and PathGG to predict time to BCR (a, b) and Mets (c, d) in all samples. KM shows that HDDA10 can better predict BCR- and Mets-free survival. High HDDA10 are defined as patients with HDDA10 score greater than 0.5 and low otherwise
Fig. 4.
Kaplan–Meier curves of the HDDA10 and primary pathological Gleason score in all samples to predict time to BCR and Mets. KM curves of HDDA10 and Primary PathGG to predict time to BCR (a, b) and Mets (c, d) in GG2-3 samples. KM shows that HDDA10 can better predict BCR- and Mets-free survival in the background of GG2–3. High HDDA10 are defined as patients with HDDA10 score greater than 0.5 and low otherwise
Association and comparison of HDDA10 to clinical–pathological parameters
Univariate and multivariate analysis using Cox regression models for the case cohort was conducted across the validation set (n = 219) to test for the effect of each variable as well as dependencies among these variables. In univariate analysis, HDDA10, extra prostatic extension (EPE), seminal vesicle invasion (SVI) were statistically significant predictors of BCR. HDDA10, Gleason Grade Groups (GG), SVI and EPE were significant predictors of clinical Mets. However, in multivariate analysis, only HDDA10 remained a significant predictor of BCR (p = 0.01; HR 2.4, 95% CI 1.2–4.7) and HDDA10 and GG were the only statistically significant predictors of Mets after adjusting for post-RP treatment (p = 0.005; HR 2.775, 95% CI 1.35–5.704) and (p = 0.04; HR 1.466, 95% CI 1.016–2.115), respectively (Table 1).
Table 1.
Univariable and multivariable hazard ratios for HDDA10 and clinico-pathological variables
| Clinical endpoint | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazards ratio (95% CI) | P value | Hazards ratio (95% CI) | P value | |
| BCR | ||||
| HDDA10 | 2.08 (1.2–3.6) | 0.008 | 2.406 (1.228–4.715) | 0.010501 |
| log (PSA) | 1.159 (0.846–1.588) | 0.359 | 1.252 (0.869–1.802) | 0.227456 |
| pathGS | 1.272 (0.978–1.655) | 0.073 | 1.242 (0.814–1.896) | 0.314773 |
| SVI | 1.939 (1.144–3.289) | 0.014 | 1.837 (0.817–4.128) | 0.140995 |
| ECE | 2.067 (1.231–3.471) | 0.006 | 1.901 (0.799–4.523) | 0.146151 |
| SMS | 1.05 (0.633–1.741) | 0.85 | 1.487 (0.812–2.721) | 0.198775 |
| AdjRTx | 0.784 (0.338–1.818) | 0.57 | 0.447 (0.161–1.244) | 0.122971 |
| AdjHTx | 1.157 (0.68–1.968) | 0.591 | 0.457 (0.183–1.142) | 0.093919 |
| Mets | ||||
| HDDA10 | 3.36 (1.72–6.56) | 0.00036 | 2.775 (1.35–5.704) | 0.005509 |
| log (PSA) | 1.218 (0.847–1.753) | 0.288 | 1.185 (0.775–1.811) | 0.432542 |
| pathGS | 1.661 (1.262–2.186) | 0.000298 | 1.466 (1.016–2.115) | 0.041059 |
| SVI | 2.032 (1.16–3.56) | 0.013 | 1.64 (0.783–3.434) | 0.189341 |
| ECE | 2.468 (1.399–4.355) | 0.002 | 1.598 (0.751–3.4) | 0.223611 |
| SMS | 1.016 (0.584–1.77) | 0.954 | 1.285 (0.653–2.53) | 0.467321 |
| AdjRTx | 1.261 (0.53–2.997) | 0.6 | 0.67 (0.216–2.08) | 0.488643 |
| AdjHTx | 2.083 (1.179–3.68) | 0.011 | 0.928 (0.419–2.054) | 0.854032 |
When limiting the univariate and multivariate analyses to patients with GG2–3, HDDA10 remained the most significant predictor of BCR and Mets compared to any other clinical or pathological parameter. Of note, HDDA10 remained the sole significant predictor of Mets on multivariate analysis (p = 0.01; HR 7.546, 95% CI 1.518–37.497) (Table 2).
Table 2.
Univariate and multivariate hazard ratios for HDDA10 and clinical–pathological variables in the GS7 subgroup
| Clinical endpoint | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazards ratio (95% CI) | P value | Hazards ratio (95% CI) | P value | |
| BCR | ||||
| HDDA10 | 4.715 (1.929–11.525) | 0.000672 | 15.853 (2.865–87.716) | 0.001546 |
| log (PSA) | 1.532 (0.984–2.386) | 0.058848 | 2.137 (1.204–3.794) | 0.009492 |
| SVI | 2.682 (1.234–5.828) | 0.012708 | 3.242 (0.657–15.997) | 0.148746 |
| ECE | 2.687 (1.235–5.846) | 0.012715 | 2.122 (0.558–8.073) | 0.26979 |
| SMS | 0.695 (0.322–1.503) | 0.355553 | 2.423 (0.651–9.023) | 0.187017 |
| AdjRTx | 0.707 (0.205–2.439) | 0.58371 | 0.936 (0.173–5.075) | 0.938893 |
| AdjHTx | 2.168 (0.944–4.978) | 0.068205 | 1.657 (0.637–4.31) | 0.30044 |
| Mets | ||||
| HDDA10 | 6.198 (1.732–22.177) | 0.005 | 7.546 (1.518–37.497) | 0.013488 |
| log (PSA) | 1.565 (0.993–2.467) | 0.053498 | 1.762 (0.942–3.294) | 0.076183 |
| SVI | 2.62 (1.114–6.163) | 0.027348 | 1.897 (0.449–8.011) | 0.383743 |
| ECE | 3.157 (1.319–7.555) | 0.009817 | 2.312 (0.673–7.943) | 0.183151 |
| SMS | 0.862 (0.358–2.076) | 0.741102 | 1.934 (0.514–7.272) | 0.328931 |
| AdjRTx | 0.869 (0.212–3.552) | 0.844732 | 1.874 (0.219–16.064) | 0.566541 |
| AdjHTx | 2.821 (1.16–6.864) | 0.022235 | 2.147 (0.644–7.161) | 0.213571 |
These results document that HDDA10 has a significant prognostic value across different clinical endpoints and in patients likely amenable for active surveillance and deferred treatment.
Combined HDDA10 and Gleason score
We used logistic regression to combine HDDA10 and GG to produce HDDA + GG. The HDDA + GG score had slight improvement to the metastasis-predictive power (AUC 0.71 (0.65–0.78), p = 2.9e– 7) and BCR (AUC 0.62 (0.55–0.7), p = 1.9e–3).
Discussion
Predicting prostate cancer progression or GG upgrading from needle biopsy tissue sampling remains a challenge using current clinical and pathological parameters. Disease progression and the development of metastatic dissemination, remain one of the most important issues still to be resolved in cancer. In the current era of PSA screening, this issue has become of increased significance where over diagnosis of small and potentially indolent tumors has reached alarming levels. Currently, reliable distinction between indolent and aggressive prostate cancer prior to implementing definite treatment is not achievable based on pathologic and clinical parameters alone. Expression profile studies have characterized numerous potential biomarkers and gene signatures related to PCa prognosis or progression. However, to date, none has been translated into clinical practice, likely due to the lack of reproducibility or because it is not robust above current clinical and pathological parameters. In addition, most of those signatures did not factor the issue of tumor heterogeneity. In this study, we have refined and validated a previously characterized 10-gene model with prognostic implication to patients with prostate cancer, especially in those with low or intermediate Gleason score, potentially candidates for active surveillance (GG1, 2 and 3).
Although, the initial gene signature was identified in relation to ERG gene rearrangements which have been suggested to represent a unique molecular subtype of PCa, it documented non-overlap and superiority above ERG status suggesting its ability to characterize downstream pathways of ERG and not ERG itself. The 10-gene signature was characterized through a combination of computational analysis and system biology studies and validated on several public well-annotated and large cohorts. Herein, we refine and validate this gene classifier referred to as HDDA10 and document its added prognostic value far beyond those obtained by other clinical and pathological parameters. The HDDA10 was trained on a case–control cohort enriched with metastasis events and was validated on an independent cohort with a case-cohort design. Both cohorts are high-risk RP samples, but the training cohort is more enriched with BCR and metastasis events given the nature of the cohort.
Our HDDA10 model was able to retain its prognostic value compared to clinical and pathological parameters in relation to multiple clinical endpoints (biochemical recurrence and clinical metastasis) and at times was the only predictor of such clinical endpoint in multivariate analysis, taking into account, the most powerful pathological predictor (GG) which is the main player in current daily practice. More interesting, was the superior added prognostic value for the HDDA10 documented in the subgroup of patients with GG2 & 3, whom likely to benefit the most of such signatures in reassuring them more reliably that they are better suited for active surveillance and as not likely to harbor higher grade disease that has not been sampled or misclassified on needle biopsy pathological examination. Given the higher frequency of patients presenting with GG2 & 3, and the challenges of treatment decision in this subgroup (such as active surveillance), we believe that HDDA10 would be of great clinical value to aid decision treatment and overcome these challenges.
Recently, there have been few ‘signatures’ in the pipeline that are currently being tested and validated to discriminate aggressive from indolent disease, including Oncotype DX Prostate and methylation signatures. However, to our knowledge, none has shown more robustness in the subgroup of patients with GG1 & 2 as HDDA10.
Given the heterogeneous and multifactorial nature of PCa, it is likely that different signatures will demonstrate variable prognostic capability in different risk groups depending on the clinical endpoint being assessed. Therefore, it is not expected to have one superior signature for all patients group (low, intermediate and high risk) and cohorts (surgical vs. radiotherapy or hormonal therapy). It is more likely that individual prognostic signature will depend on specific clinical scenarios being assessed.
An important issue herein is the potential utilization of such signatures to reclassify PCa based on biological behavior rather than morphology alone.
In summary, the HDDA0 molecular gene classifier is robust and of added prognostic value in different clinical endpoint, specifically in low and intermediate-risk patient groups likely to select active surveillance as a treatment option. The HDDA10 classifier should be prospectively tested in active surveillance cohorts to validate its prognostic value in allowing more men to safely be included in such programs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported in part by the Prostate Cancer Foundation Young Investigator Award (T.A.B). This work was also supported by Prostate cancer Canada and is proudly funded by the Movember Foundation-Grant #B2013-01. This work was also supported in part by the GAP1 Movember Tissue Biomarker Project.
Author contributions
HAO drafted the paper, MA carried out bioinformatics and computational analysis, TAB is responsible for outline, study design and supervision of work, all authors have approved the submitted and published versions.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest in regards to this manuscript.
Ethical approval
This research was performed in compliance with all ethical standards and approved by the U Calgary ethics board.
Footnotes
Statement of Significance: The study provides evidence of employing molecular classifier to assist physicians in better stratifying men with prostate cancer into different risk groups.
References
- Andren O, Fall K, Andersson SO, Rubin MA, Bismar TA, Karlsson M, Johansson JE, Mucci LA (2007) MUC-1 gene is associated with prostate cancer death: a 20-year follow-up of a population-based study in Sweden. Br J Cancer 97(6):730–734. doi: 10.1038/sj.bjc.6603944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, Berney D, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter V, De Bono JS, Scardino P, Cuzick J, Cooper CS (2008) Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 27(3):253–263. 10.1038/sj.onc.1210640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Silva JD, Ribeiro FR, Rodrigues A, Cruz R, Martins AT, Jeronimo C, Henrique R, Teixeira MR (2011) Relative 8q gain predicts disease-specific survival irrespective of the TMPRSS2-ERG fusion status in diagnostic biopsies of prostate cancer. Genes Chromosom Cancer 50(8):662–671. 10.1002/gcc.20888 [DOI] [PubMed] [Google Scholar]
- Bergé L, Bouveyron C, Girard S et al (2012) HDclassif: An R package for model-based clustering and discriminant analysis of high-dimensional data. J Stat Softw 46(6):1–2922837731 [Google Scholar]
- Bismar TA, Demichelis F, Riva A, Kim R, Varambally S, He L, Kutok J, Aster JC, Tang J, Kuefer R, Hofer MD, Febbo PG, Chinnaiyan AM, Rubin MA (2006) Defining aggressive prostate cancer using a 12-gene model. Neoplasia 8(1):59–68. 10.1593/neo.05664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismar TA, Dolph M, Teng LH, Liu S, Donnelly B (2012) ERG protein expression reflects hormonal treatment response and is associated with Gleason score and prostate cancer specific mortality. Eur J Cancer 48(4):538–546. 10.1016/j.ejca.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Bismar TA, Alshalalfa M, Petersen LF, Teng LH, Gerke T, Bakkar A, Al-Mami A, Liu S, Dolph M, Mucci LA, Alhajj R (2013) Interrogation of ERG gene rearrangements in prostate cancer identifies a prognostic 10-gene signature with relevant implication to patients’ clinical outcome. BJU Int. 10.1111/bju.12262 [DOI] [PubMed] [Google Scholar]
- Bul M, van den Bergh RCN, Zhu X, Rannikko A, Vasarainen H, Bangma CH, Schröder FH, Roobol MJ (2012) Outcomes of initially expectantly managed patients with low or intermediate risk screen-detected localized prostate cancer. BJU Int 110(11):1672–1677. 10.1111/j.1464-410X.2012.11434.x [DOI] [PubMed] [Google Scholar]
- Carter HB, Isaacs WB (2004) Improved biomarkers for prostate cancer: a definite need. J Natl Cancer Inst 96(11):813–815 [DOI] [PubMed] [Google Scholar]
- Cazares LH, Drake RR, Esquela-Kirscher A, Lance RS, Semmes OJ, Troyer DA (2010) Molecular pathology of prostate cancer. Cancer Biomark Sect A Dis Markers 9(1–6):441–459. 10.3233/CBM-2011-0181 [DOI] [PubMed] [Google Scholar]
- Darnel AD, Behmoaram E, Vollmer RT, Corcos J, Bijian K, Sircar K, Su J, Jiao J, Alaoui-Jamali MA, Bismar TA (2009) Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochemical failure in prostate cancer. Clin Cancer Res 15(4):1376–1383. 10.1158/1078-0432.CCR-08-1789 [DOI] [PubMed] [Google Scholar]
- Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, Hoshida Y, Mosquera JM, Pawitan Y, Lee C, Adami HO, Mucci LA, Kantoff PW, Andersson SO, Chinnaiyan AM, Johansson JE, Rubin MA (2007) TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 26(31):4596–4599. 10.1038/sj.onc.1210237 [DOI] [PubMed] [Google Scholar]
- El Sheikh SS, Romanska HM, Abel P, Domin J, Lalani el N (2008) Predictive value of PTEN and AR coexpression of sustained responsiveness to hormonal therapy in prostate cancer—a pilot study. Neoplasia 10(9):949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JI, Feng Z, Trock BJ, Pierorazio PM (2012) Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 61(5):1019–1024. 10.1016/j.eururo.2012.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, Bergstralh EJ, Kollmeyer T, Fink S, Haddad Z, Zimmermann B, Sierocinski T, Ballman KV, Triche TJ, Black PC, Karnes RJ, Klee G, Davicioni E, Jenkins RB (2013) Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One 8(6):e66855. 10.1371/journal.pone.0066855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall K, Garmo H, Andren O, Bill-Axelson A, Adolfsson J, Adami HO, Johansson JE, Holmberg L (2007) Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst 99(7):526–532. 10.1093/jnci/djk110 [DOI] [PubMed] [Google Scholar]
- Glinsky GV, Glinskii AB, Stephenson AJ, Hoffman RM, Gerald WL (2004) Gene expression profiling predicts clinical outcome of prostate cancer. J Clin Invest 113(6):913–923. 10.1172/JCI20032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg A, Mostafavi S, Quon G, Boutros PC, Morris QD (2011) Unsupervised detection of genes of influence in lung cancer using biological networks. Bioinformatics 27(22):3166–3172. 10.1093/bioinformatics/btr533 [DOI] [PubMed] [Google Scholar]
- Halvorsen OJ, Haukaas SA, Akslen LA (2003) Combined loss of PTEN and p27 expression is associated with tumor cell proliferation by Ki-67 and increased risk of recurrent disease in localized prostate cancer. Clin Cancer Res 9(4):1474–1479 [PubMed] [Google Scholar]
- Iremashvili V, Soloway MS, Pelaez L, Rosenberg DL, Manoharan M (2013) Comparative validation of nomograms predicting clinically insignificant prostate cancer. Urology 81(6):1202–1208 [DOI] [PubMed] [Google Scholar]
- Karnes RJ, Bergstralh EJ, Davicioni E, Ghadessi M, Buerki C, Mitra AP, Crisan A, Erho N, Vergara IA, Lam LL, Carlson R, Thompson DJS, Haddad Z, Zimmermann B, Sierocinski T, Triche TJ, Kollmeyer T, Ballman KV, Black PC, Klee GG, Jenkins RB (2013) Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J Urol 190(6):2047–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibel AS (2007) TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, Hoshida Y, Mosquera JM, Pawitan Y, Lee C, Adami HO, Mucci LA, Kantoff PW, Andersson SO, Chinnaiyan AM, Johansson JE, Rubin MA, Department of Pathology, Brigham and Women’s Hospital, Boston. Urol Oncol 25 (5):448–449 [DOI] [PubMed] [Google Scholar]
- Li Y, Su J, Dingzhang X, Zhang J, Yoshimoto M, Liu S, Bijian K, Gupta A, Squire JA, Alaoui Jamali MA, Bismar TA (2011) PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J Pathol. 10.1002/path.2855 [DOI] [PubMed] [Google Scholar]
- Mucci LA, Pawitan Y, Demichelis F, Fall K, Stark JR, Adami HO, Andersson SO, Andren O, Eisenstein AS, Holmberg L, Huang W, Kantoff PW, Perner S, Stampfer MJ, Johansson JE, Rubin MA (2008) Nine-gene molecular signature is not associated with prostate cancer death in a watchful waiting cohort. Cancer Epidemiol Biomark Prev 17(1):249–251. 10.1158/1055-9965.EPI-07-0722 [DOI] [PubMed] [Google Scholar]
- Odom BD, Mir MC, Hughes S, Senechal C, Santy A, Eyraud R, Stephenson AJ, Ylitalo K, Miocinovic R (2014) Active surveillance for low-risk prostate cancer in African American men: a multi-institutional experience. Urology 83(2):364–368. 10.1016/j.urology.2013.09.038 [DOI] [PubMed] [Google Scholar]
- Piccolo SR, Sun Y, Campbell JD, Lenburg ME, Bild AH, Johnson WE (2012) A single-sample microarray normalization method to facilitate personalized-medicine workflows. Genomics 100(6):337–344. 10.1016/j.ygeno.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierorazio PM, Walsh PC, Partin AW, Epstein JI (2013) Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 111(5):753–760. 10.1111/j.1464-410X.2012.11611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AH, Attard G, Ambroisine L, Fisher G, Kovacs G, Brewer D, Clark J, Flohr P, Edwards S, Berney DM, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter VE, Scardino PT, Cuzick J, de Bono JS, Cooper CS (2010) Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer 102(4):678–684. 10.1038/sj.bjc.6605554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sboner A, Demichelis F, Calza S, Pawitan Y, Setlur SR, Hoshida Y, Perner S, Adami HO, Fall K, Mucci LA, Kantoff PW, Stampfer M, Andersson SO, Varenhorst E, Johansson JE, Gerstein MB, Golub TR, Rubin MA, Andren O (2010) Molecular sampling of prostate cancer: a dilemma for predicting disease progression. BMC Med Genomics 3:8. 10.1186/1755-8794-3-8 [DOI] [PMC free article] [PubMed]
- Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM (2013) Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol [DOI] [PMC free article] [PubMed]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18(1):11–22 10.1016/j.ccr.2010.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310(5748):644–648 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.