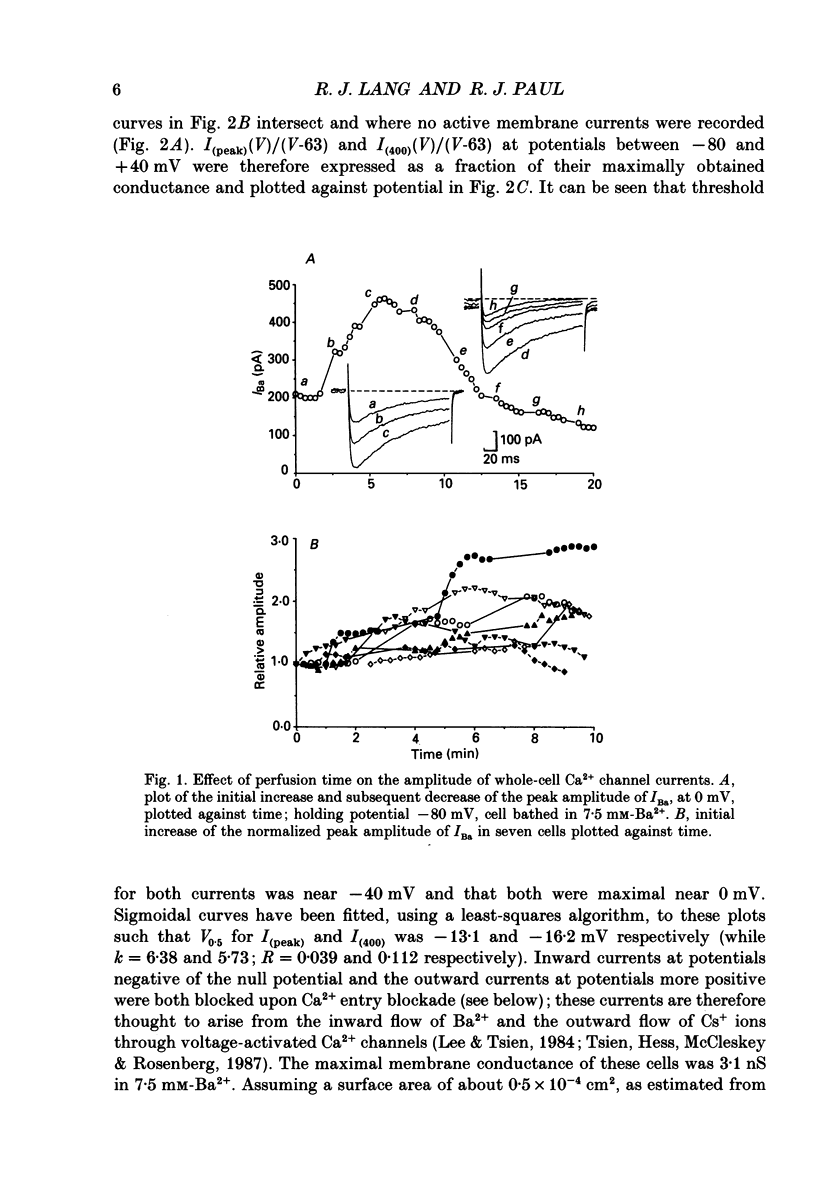
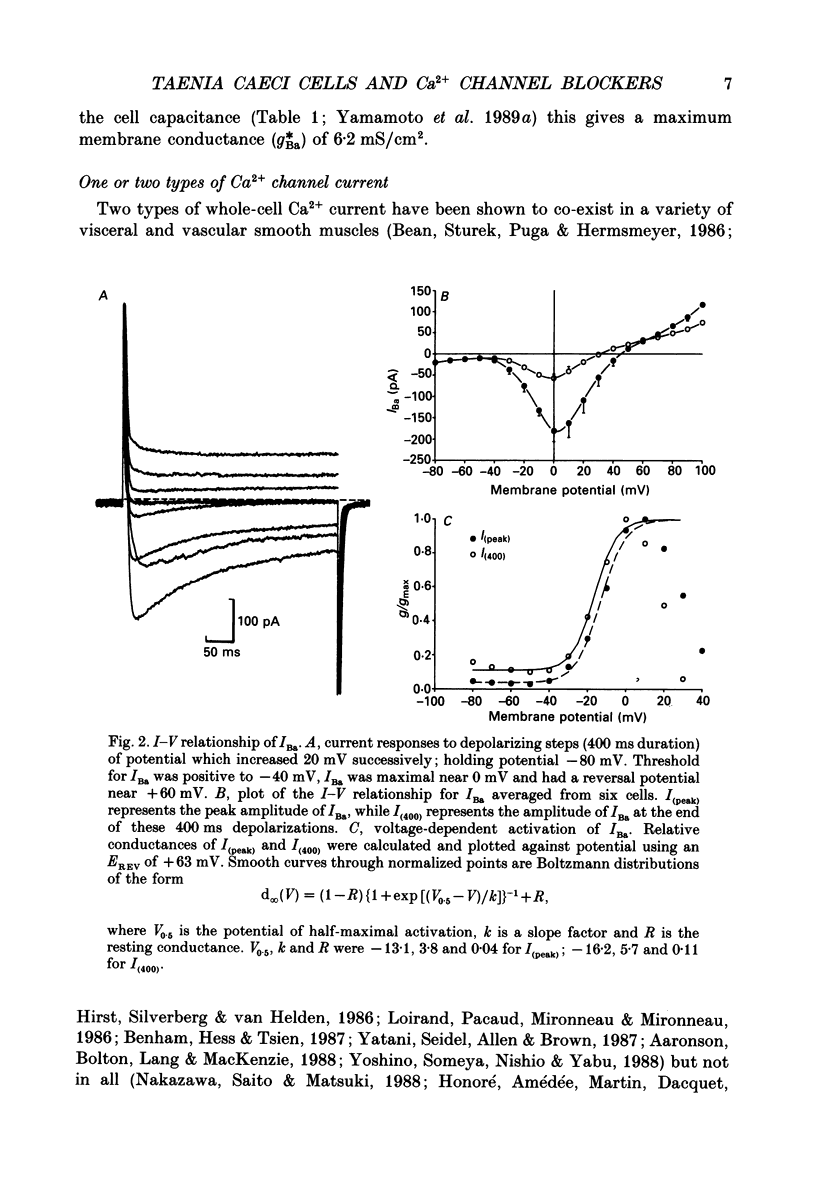
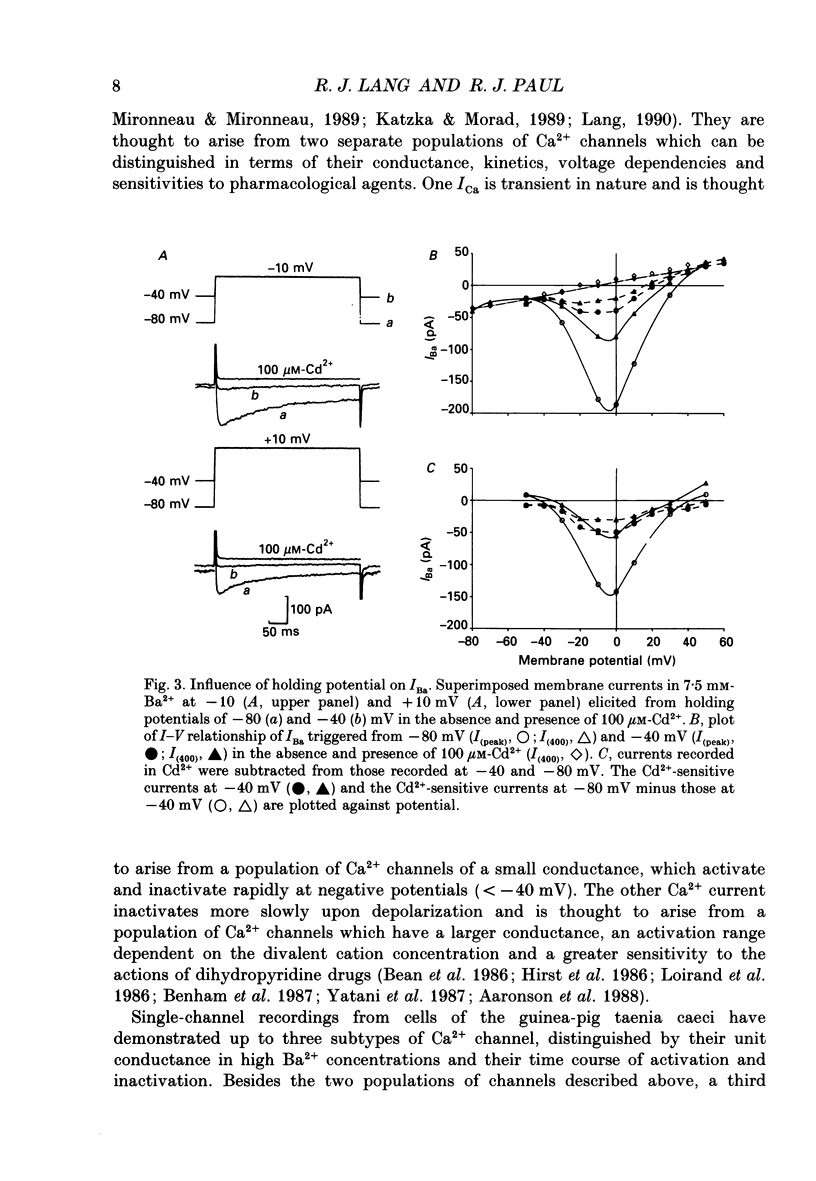
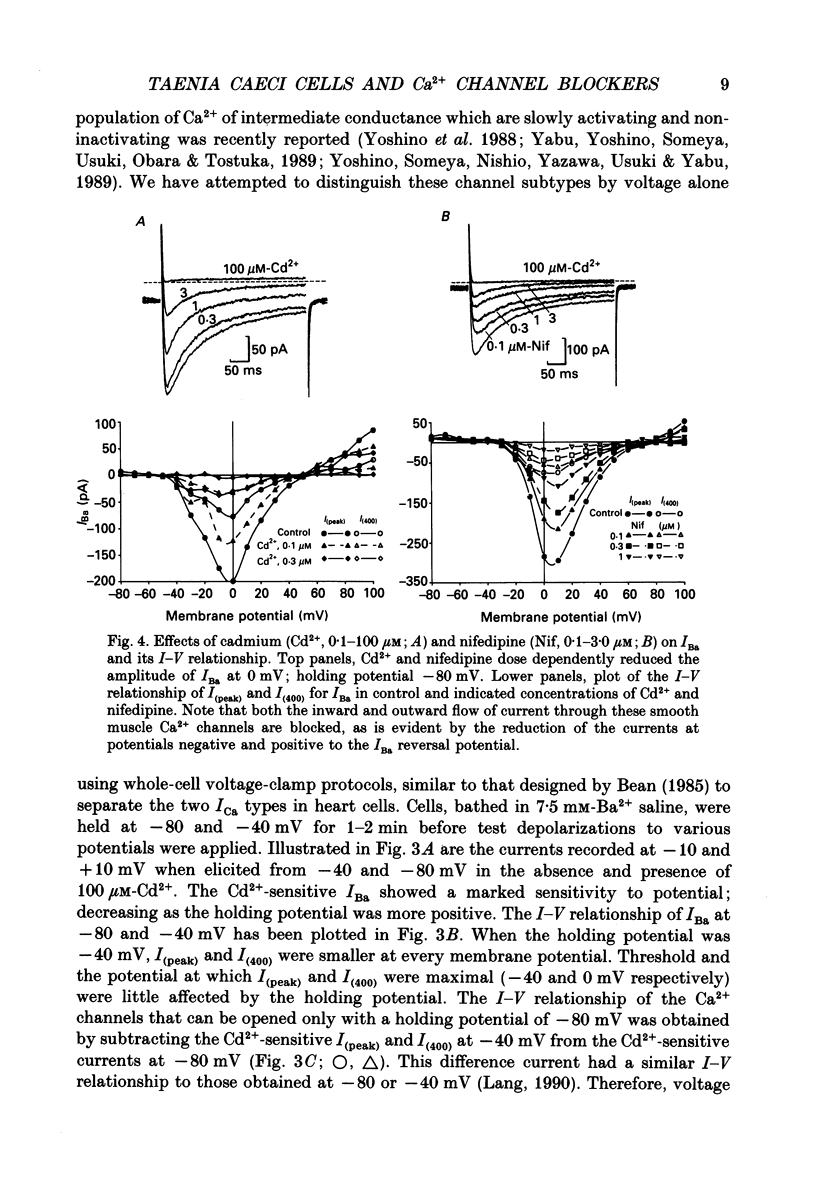
Abstract
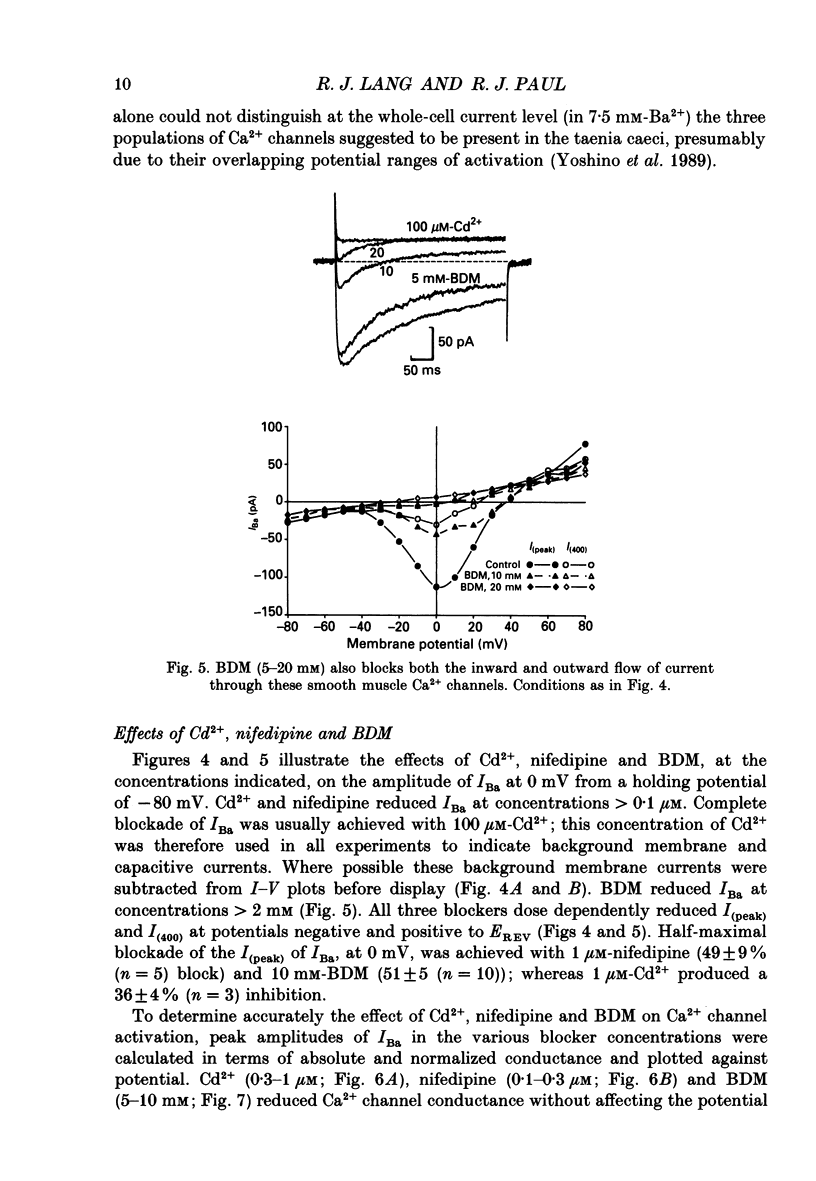
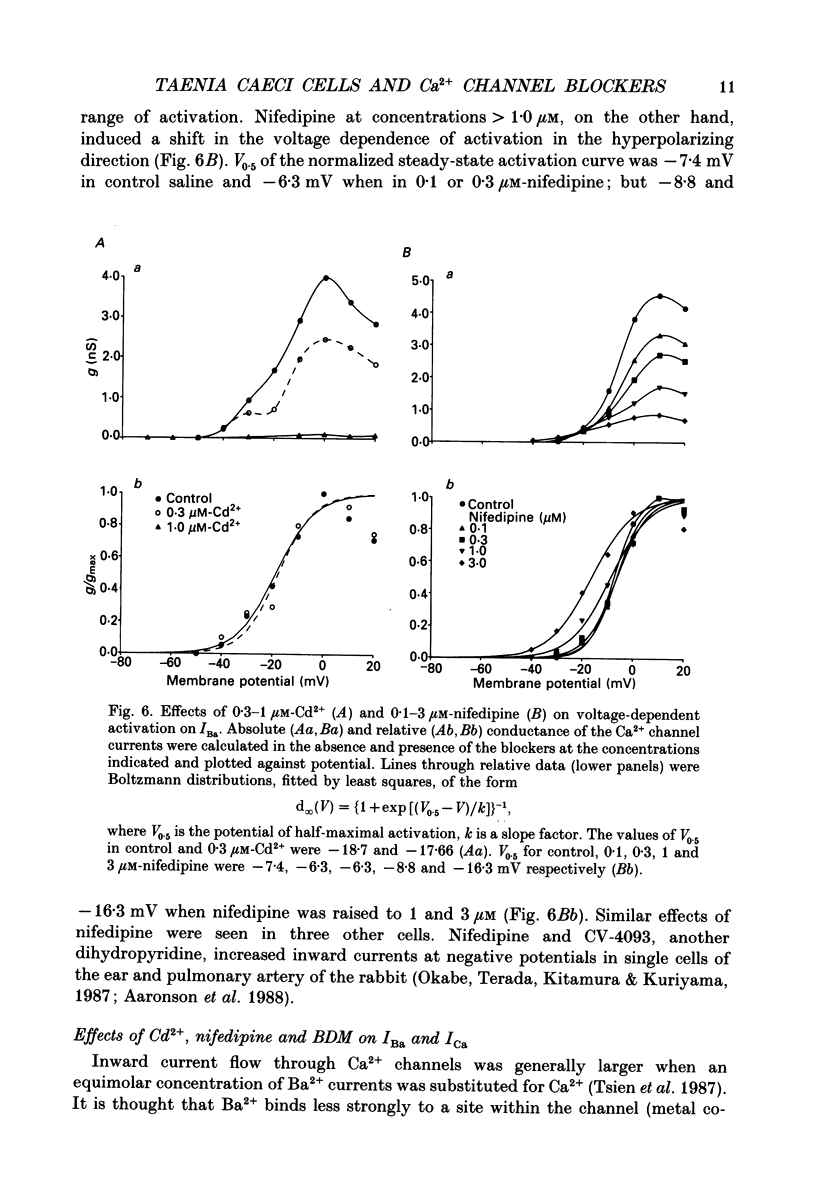
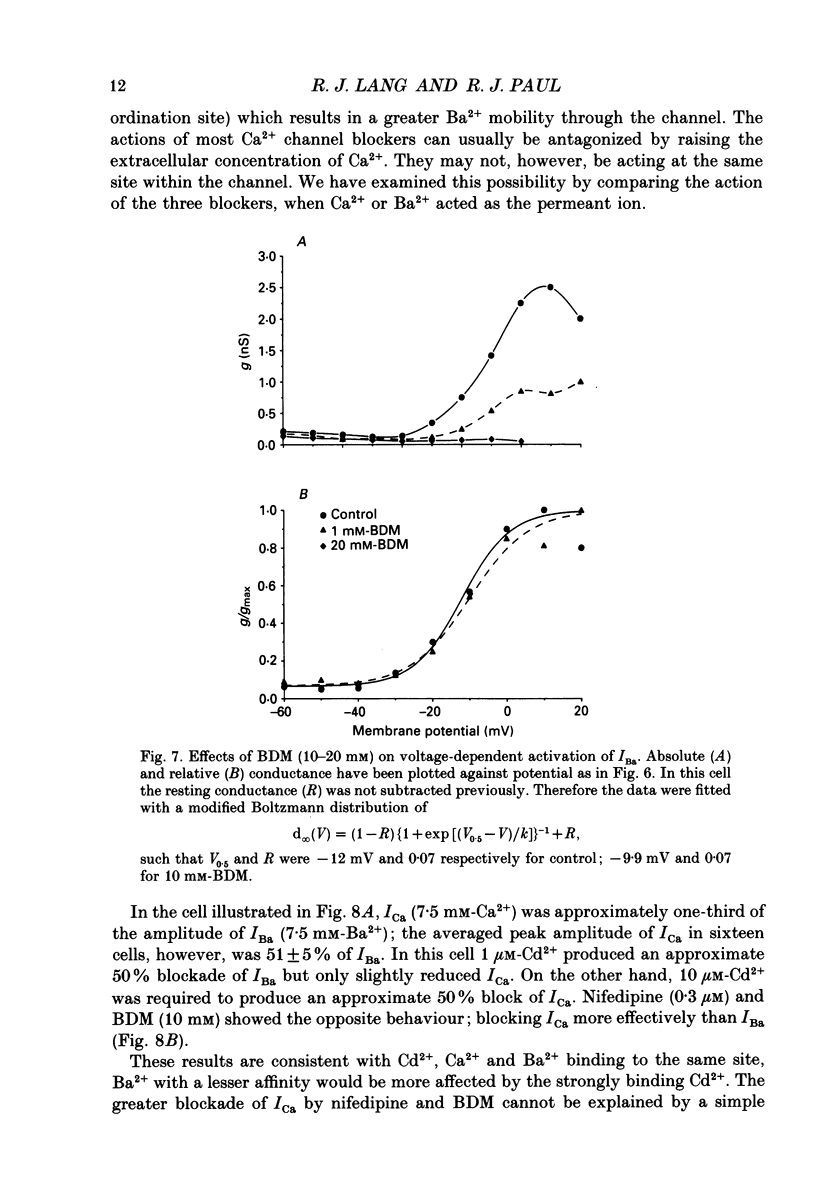
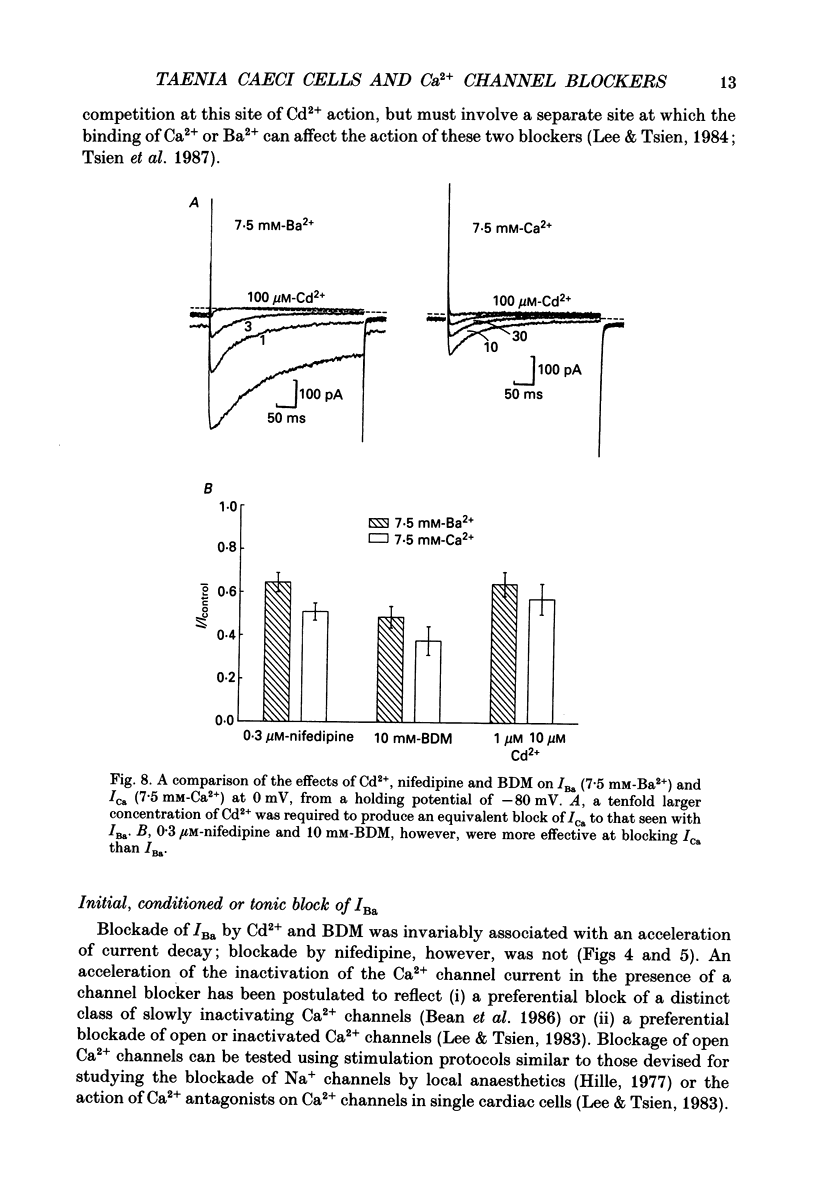
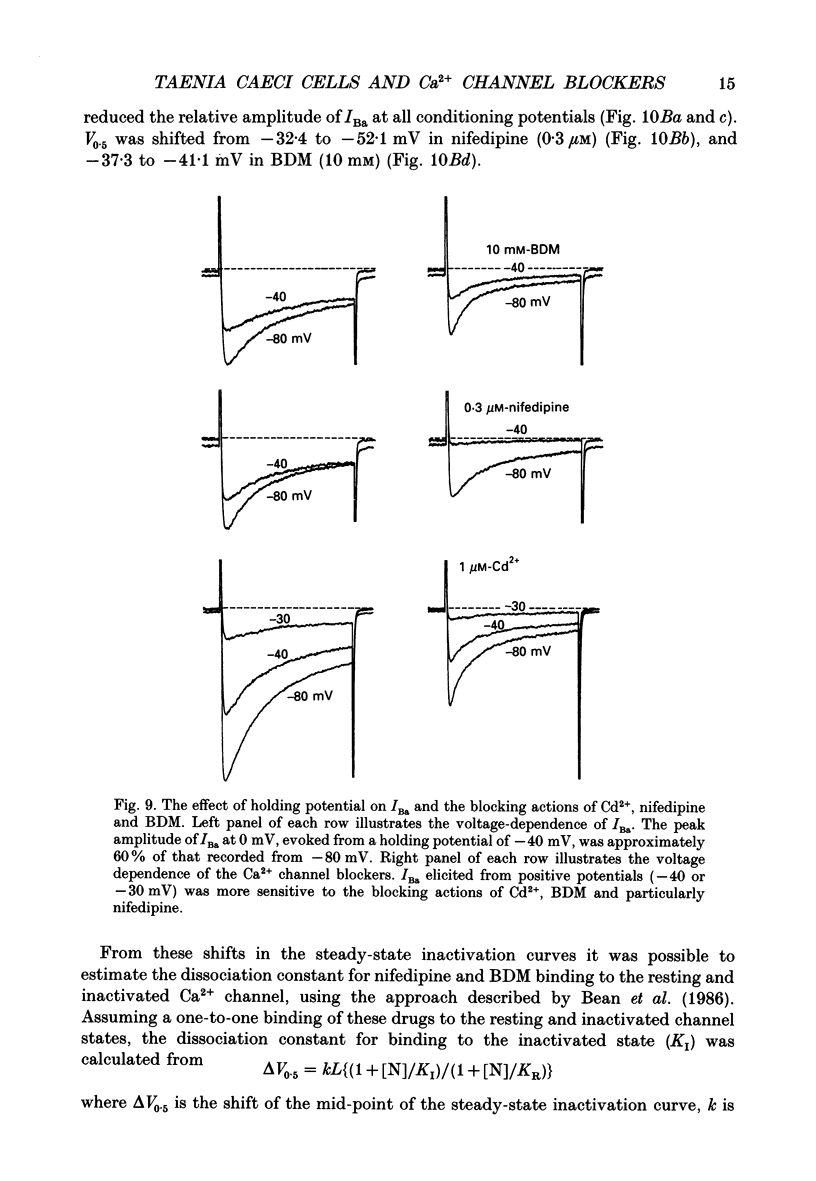
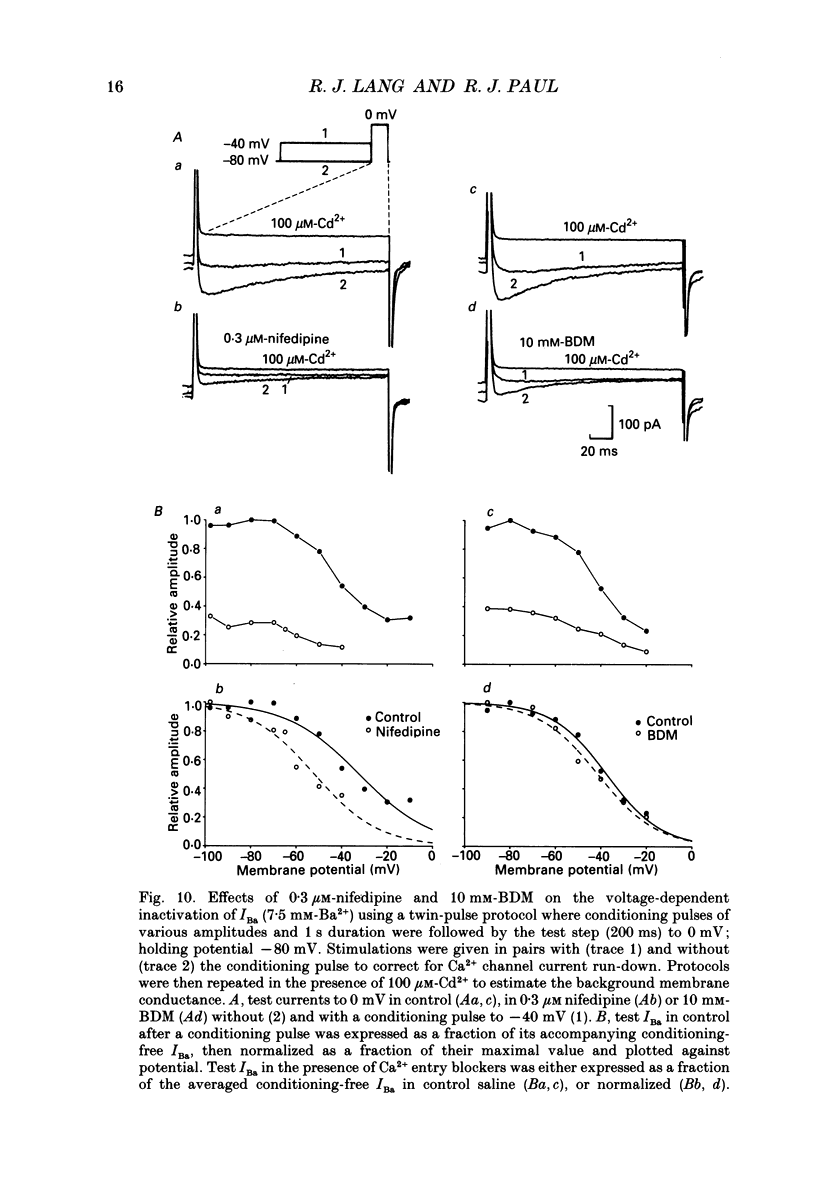
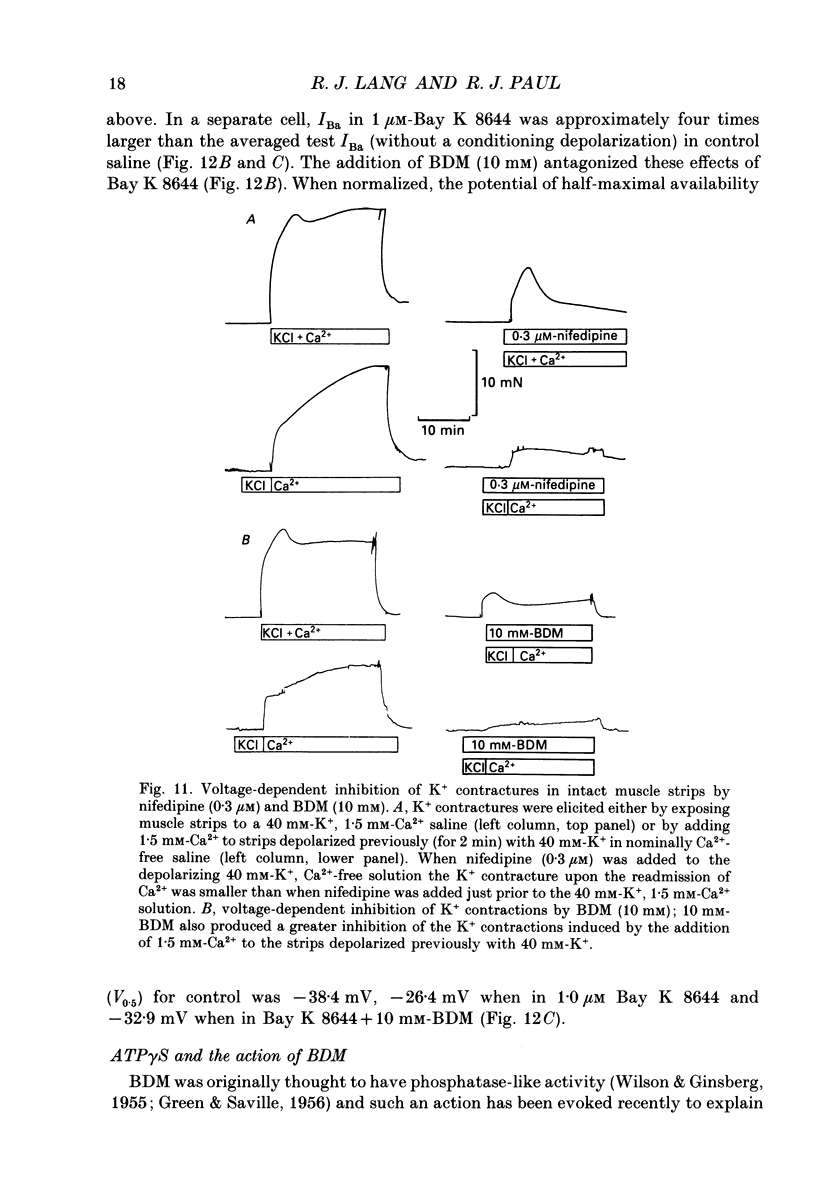
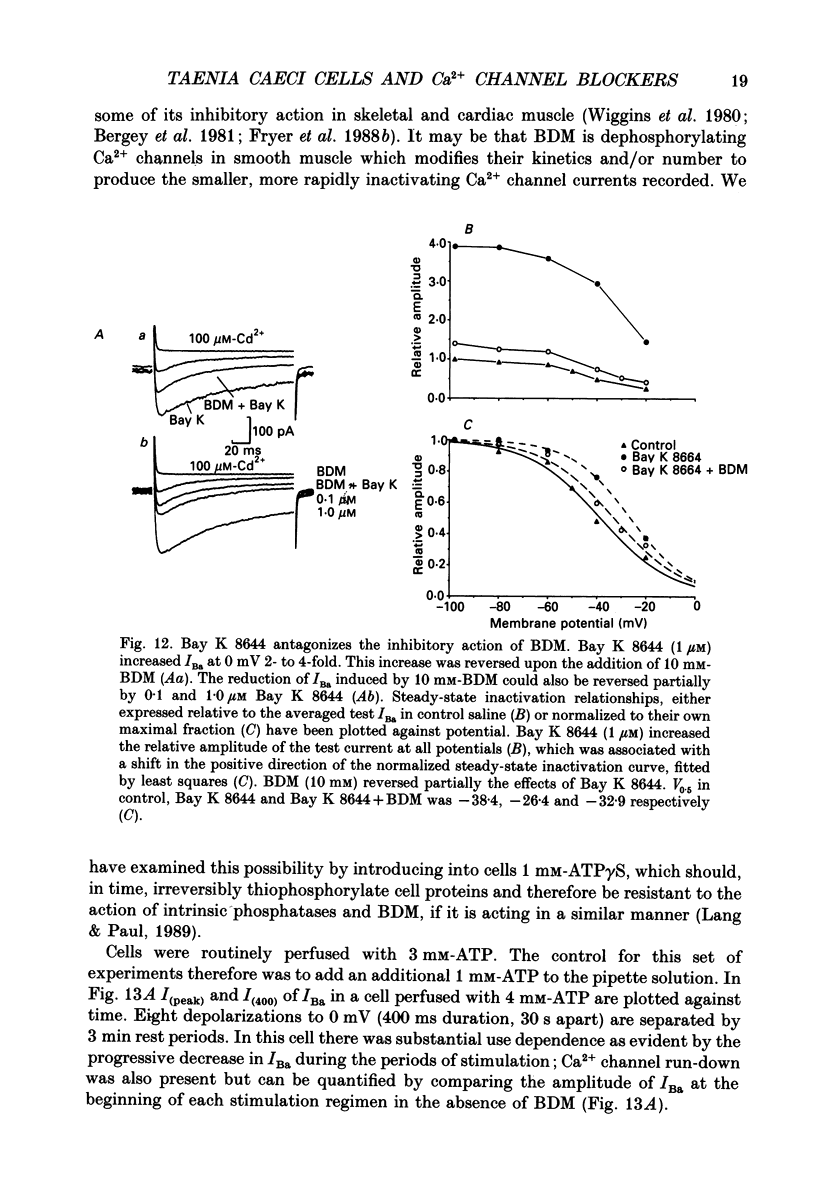
1. The inhibitory actions of cadmium (Cd2+), nifedipine and 2,3-butanedione monoxime (BDM) on whole-cell Ca2+ channel currents in single cells of the guinea-pig taenia caeci were investigated using a single-electrode whole-cell voltage-clamp technique. 2. Calcium channel currents were isolated using pipette solutions containing Cs+, tetraethylammonium and ATP (3 mM). Ca2+ or Ba2+ (7.5 mM) in the bathing solution acted as the charge carrier during inward current flow. Ca2+ channel currents in 7.5 mM-Ba2+ (IBa) were recorded at potentials positive to -40 mV, were maximal near 0 mV and reversed near +60 mV. Ca2+ channel activation showed a sigmoidal relationship with potential, which was half-maximal at -13 mV. 3. Both the inward and outward flow of current was depressed and eventually blocked by 0.3-100 microM-Cd2+, 0.1-10 microM-nifedipine and 2-20 mM-BDM. Half-maximal blockade of IBa at 0 mV was achieved with approximately 3 microM-Cd2+, 1 microM-nifedipine and 10 microM-BDM. Steady-state activation curves were not affected by Cd2+ or BDM, but were shifted in the hyperpolarizing direction by nifedipine at concentrations > 1 microM. 4. Calcium channel currents in single cells and K+ contractures in intact strips were both blocked in a voltage-dependent manner. Steady-state inactivation curves (f infinity (V)) for IBa were shifted 20 mV in the hyperpolarizing direction by 0.3 microM-nifedipine and 4 mV by 10 mM-BDM. From these shifts a dissociation binding constant to inactivated Ca2+ channels for nifedipine was estimated as 78 nM, and for BDM, 5 mM. 5. At 10 microM Cd2+ produced a 43 +/- 6% (n = 3) block of the inward current at 0 mV when Ca2+ (7.5 mM) was the charge carrier (ICa), compared with the 36 +/- 3% block of IBa induced by 1 microM-Cd2+, consistent with the suggestion that Ca2+, Ba2+ and Cd2+ compete for the same binding site. In contrast, nifedipine (1 microM) and BDM (10 mM) blocked ICa more effectively than IBa. 6. Bay K 8644 (1.0 microM) increased Ca2+ channel currents two- to fourfold at all potentials due to a shift, of approximately 10 mV in the negative direction, of their activation curve and an equal shift in the positive direction of their inactivation curve. BDM (5-10 mM) could antagonize the action of Bay K 8644, shifting both curves back towards their control.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I., Bolton T. B., Lang R. J., MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol. 1988 Nov;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey J. L., Reiser J., Wiggins J. R., Freeman A. R. Oximes: 'enzymatic' slow channel antagonists in canine cardiac purkinje fibers? Eur J Pharmacol. 1981 May 8;71(2-3):307–319. doi: 10.1016/0014-2999(81)90033-9. [DOI] [PubMed] [Google Scholar]
- Dacquet C., Loirand G., Mironneau C., Mironneau J., Pacaud P. Spironolactone inhibition of contraction and calcium channels in rat portal vein. Br J Pharmacol. 1987 Nov;92(3):535–544. doi: 10.1111/j.1476-5381.1987.tb11354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer M. W., Gage P. W., Neering I. R., Dulhunty A. F., Lamb G. D. Paralysis of skeletal muscle by butanedione monoxime, a chemical phosphatase. Pflugers Arch. 1988 Jan;411(1):76–79. doi: 10.1007/BF00581649. [DOI] [PubMed] [Google Scholar]
- Fryer M. W., Neering I. R., Stephenson D. G. Effects of 2,3-butanedione monoxime on the contractile activation properties of fast- and slow-twitch rat muscle fibres. J Physiol. 1988 Dec;407:53–75. doi: 10.1113/jphysiol.1988.sp017403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987 Nov;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Potential-dependent calcium inward current in a single isolated smooth muscle cell of the guinea-pig taenia caeci. J Physiol. 1986 Nov;380:1–16. doi: 10.1113/jphysiol.1986.sp016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Saturation of calcium channels in single isolated smooth muscle cells of guinea-pig taenia caeci. J Physiol. 1988 May;399:419–436. doi: 10.1113/jphysiol.1988.sp017089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering S., Beech D. J., Bolton T. B., Lim S. P. Action of nifedipine or BAY K8644 is dependent on calcium channel state in single smooth muscle cells from rabbit ear artery. Pflugers Arch. 1988 May;411(5):590–592. doi: 10.1007/BF00582383. [DOI] [PubMed] [Google Scholar]
- Hering S., Bolton T. B., Beech D. J., Lim S. P. Mechanism of calcium channel block by D600 in single smooth muscle cells from rabbit ear artery. Circ Res. 1989 May;64(5):928–936. doi: 10.1161/01.res.64.5.928. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silverberg G. D., van Helden D. F. The action potential and underlying ionic currents in proximal rat middle cerebral arterioles. J Physiol. 1986 Feb;371:289–304. doi: 10.1113/jphysiol.1986.sp015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Amédée T., Martin C., Dacquet C., Mironneau C., Mironneau J. Calcium channel current and its sensitivity to (+) isradipine in cultured pregnant rat myometrial cells. An electrophysiological and a binding study. Pflugers Arch. 1989 Aug;414(4):477–483. doi: 10.1007/BF00585060. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Mieskes G., Trautwein W. The protein-specific phosphatase 1 antagonizes the beta-adrenergic increase of the cardiac Ca current. Pflugers Arch. 1986 Oct;407(4):461–463. doi: 10.1007/BF00652635. [DOI] [PubMed] [Google Scholar]
- Katzka D. A., Morad M. Properties of calcium channels in guinea-pig gastric myocytes. J Physiol. 1989 Jun;413:175–197. doi: 10.1113/jphysiol.1989.sp017648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. The dihydropyridine niguldipine modulates calcium and potassium currents in vascular smooth muscle cells. Br J Pharmacol. 1989 Jul;97(3):957–967. doi: 10.1111/j.1476-5381.1989.tb12037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Tiapamil reduces the calcium inward current of isolated smooth muscle cells. Dependence on holding potential and pulse frequency. Eur J Pharmacol. 1986 Aug 15;127(3):165–171. doi: 10.1016/0014-2999(86)90360-2. [DOI] [PubMed] [Google Scholar]
- Lang R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J Physiol. 1989 May;412:375–395. doi: 10.1113/jphysiol.1989.sp017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. J. The whole-cell Ca2+ channel current in single smooth muscle cells of the guinea-pig ureter. J Physiol. 1990 Apr;423:453–473. doi: 10.1113/jphysiol.1990.sp018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P. D., Burke E. P., Sanders K. M. Participation of Ca currents in colonic electrical activity. Am J Physiol. 1989 Sep;257(3 Pt 1):C451–C460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Li T., Sperelakis N., Teneick R. E., Solaro R. J. Effects of diacetyl monoxime on cardiac excitation-contraction coupling. J Pharmacol Exp Ther. 1985 Mar;232(3):688–695. [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. Evidence for two distinct calcium channels in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1986 Nov;407(5):566–568. doi: 10.1007/BF00657519. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Saito H., Matsuki N. Fast and slowly inactivating components of Ca-channel current and their sensitivities to nicardipine in isolated smooth muscle cells from rat vas deferens. Pflugers Arch. 1988 Mar;411(3):289–295. doi: 10.1007/BF00585117. [DOI] [PubMed] [Google Scholar]
- Okabe K., Terada K., Kitamura K., Kuriyama H. Selective and long-lasting inhibitory actions of the dihydropyridine derivative, CV-4093, on calcium currents in smooth muscle cells of the rabbit pulmonary artery. J Pharmacol Exp Ther. 1987 Nov;243(2):703–710. [PubMed] [Google Scholar]
- Sada H., Sada S., Sperelakis N. The calcium channel agonist, Bay K-8644, antagonizes effects of diacetyl monoxime on cardiac tissues. Can J Physiol Pharmacol. 1985 Oct;63(10):1267–1270. doi: 10.1139/y85-210. [DOI] [PubMed] [Google Scholar]
- Terada K., Kitamura K., Kuriyama H. Blocking actions of Ca2+ antagonists on the Ca2+ channels in the smooth muscle cell membrane of rabbit small intestine. Pflugers Arch. 1987 May;408(6):552–557. doi: 10.1007/BF00581155. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Hess P., McCleskey E. W., Rosenberg R. L. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- WILSON I. B., GINSBURG B. A powerful reactivator of alkylphosphate-inhibited acetylcholinesterase. Biochim Biophys Acta. 1955 Sep;18(1):168–170. doi: 10.1016/0006-3002(55)90040-8. [DOI] [PubMed] [Google Scholar]
- Wiggins J. R., Reiser J., Fitzpatrick D. F., Bergey J. L. Inotropic actions of diacetyl monoxime in cat ventricular muscle. J Pharmacol Exp Ther. 1980 Feb;212(2):217–224. [PubMed] [Google Scholar]
- Yamamoto Y., Hu S. L., Kao C. Y. Inward current in single smooth muscle cells of the guinea pig taenia coli. J Gen Physiol. 1989 Mar;93(3):521–550. doi: 10.1085/jgp.93.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Hu S. L., Kao C. Y. Outward current in single smooth muscle cells of the guinea pig taenia coli. J Gen Physiol. 1989 Mar;93(3):551–564. doi: 10.1085/jgp.93.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Seidel C. L., Allen J., Brown A. M. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987 Apr;60(4):523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]
- Yoshino M., Someya T., Nishio A., Yabu H. Whole-cell and unitary Ca channel currents in mammalian intestinal smooth muscle cells: evidence for the existence of two types of Ca channels. Pflugers Arch. 1988 Feb;411(2):229–231. doi: 10.1007/BF00582322. [DOI] [PubMed] [Google Scholar]
- Yoshino M., Someya T., Nishio A., Yazawa K., Usuki T., Yabu H. Multiple types of voltage-dependent Ca channels in mammalian intestinal smooth muscle cells. Pflugers Arch. 1989 Aug;414(4):401–409. doi: 10.1007/BF00585049. [DOI] [PubMed] [Google Scholar]


