Abstract
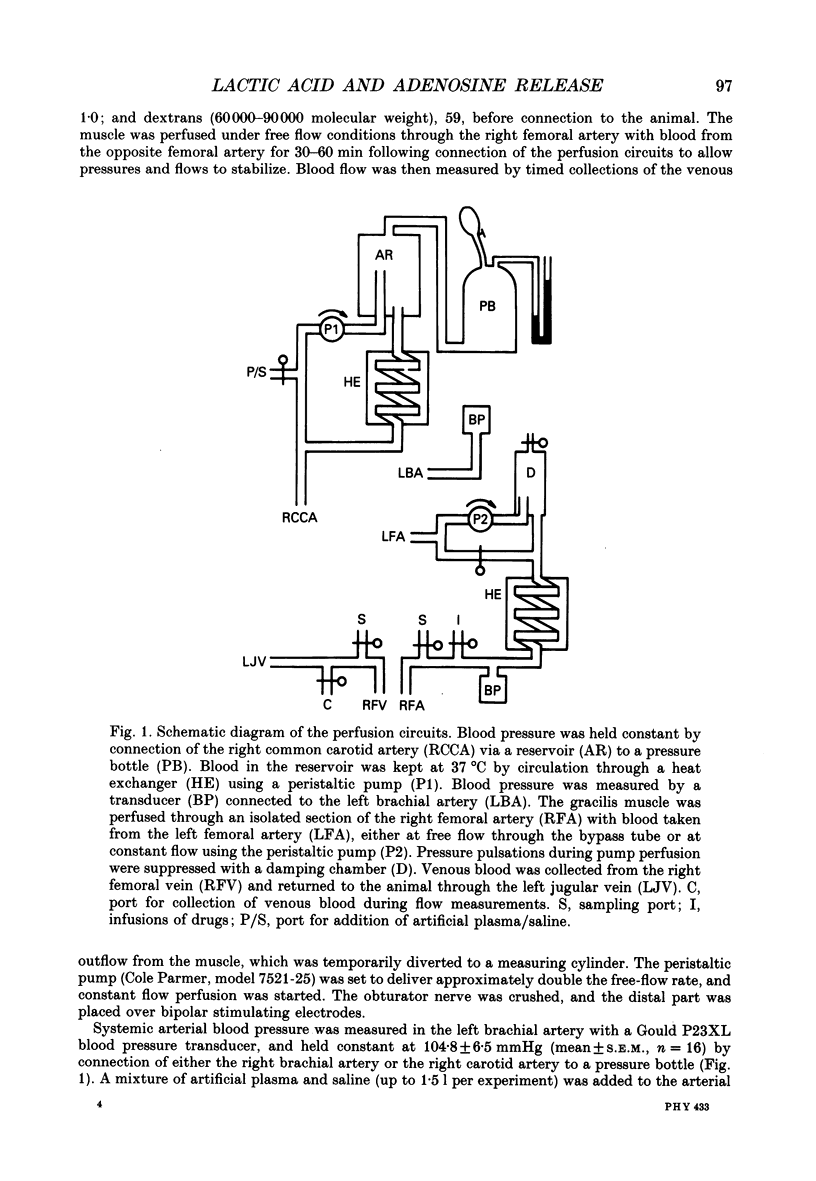
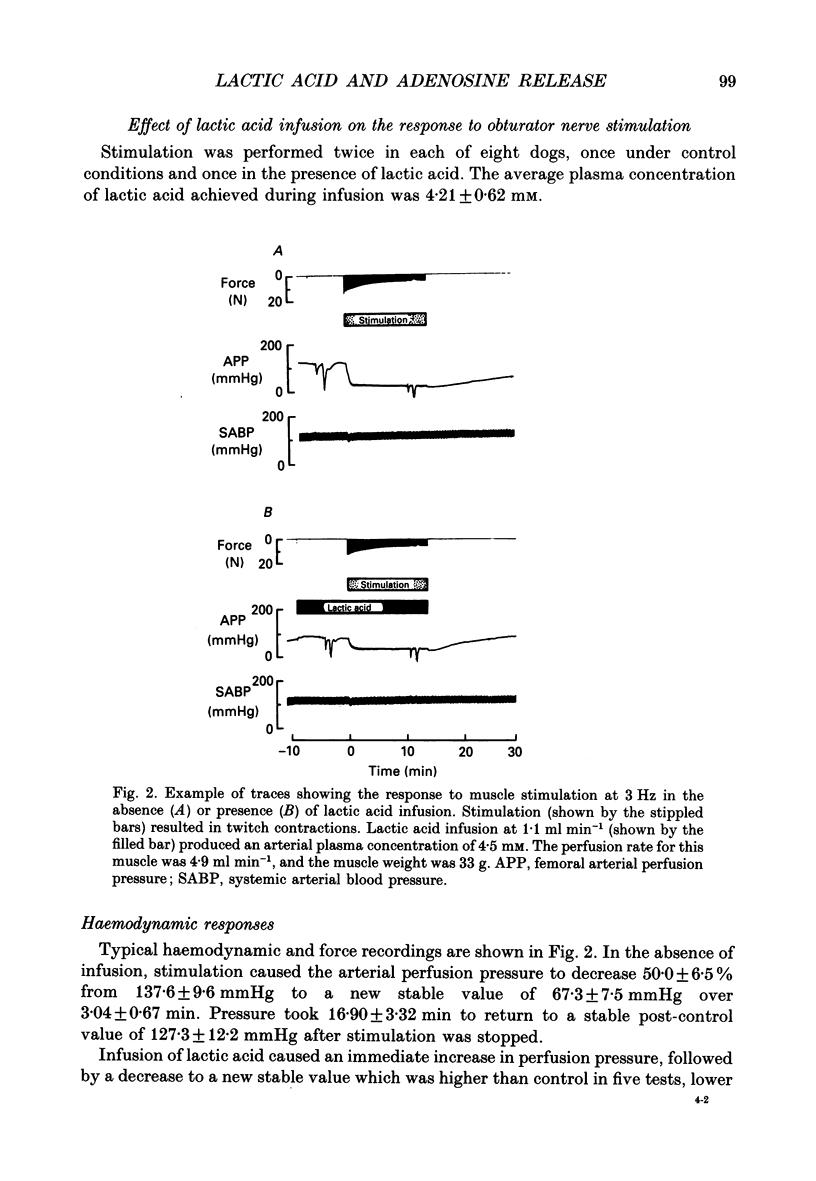
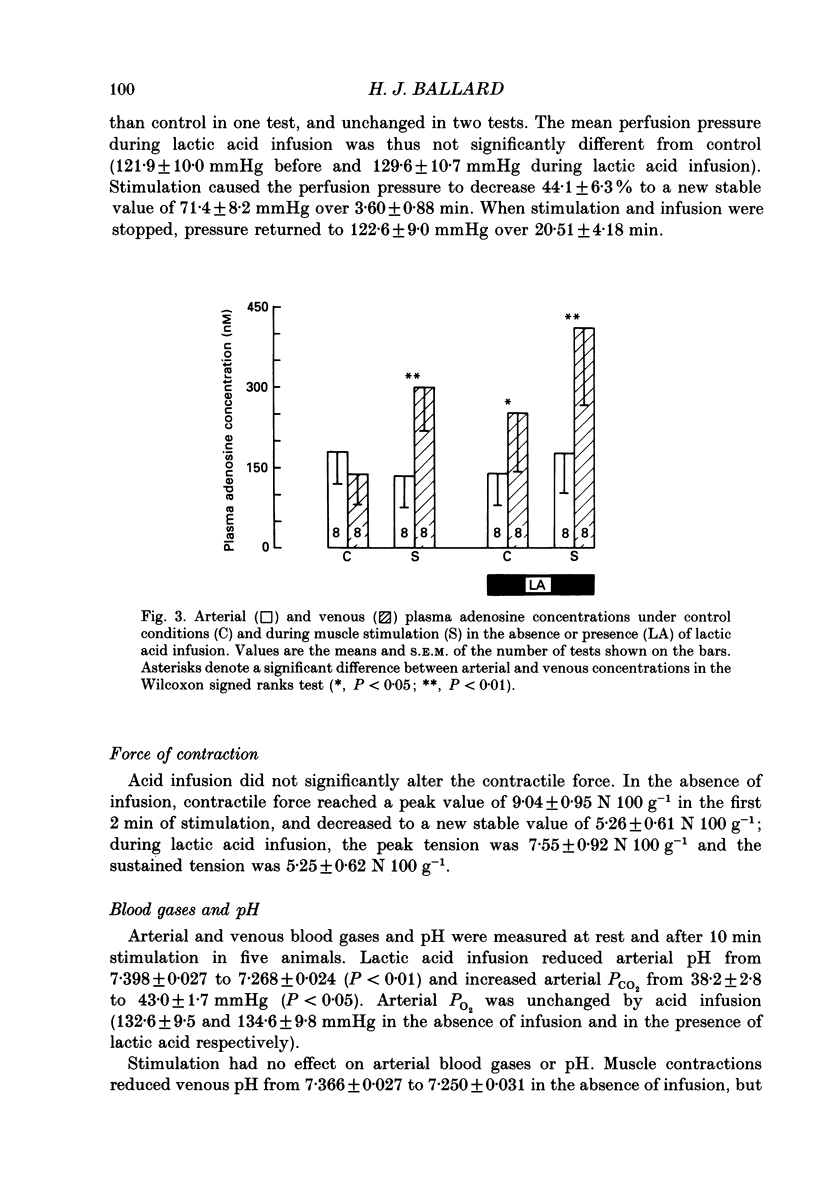
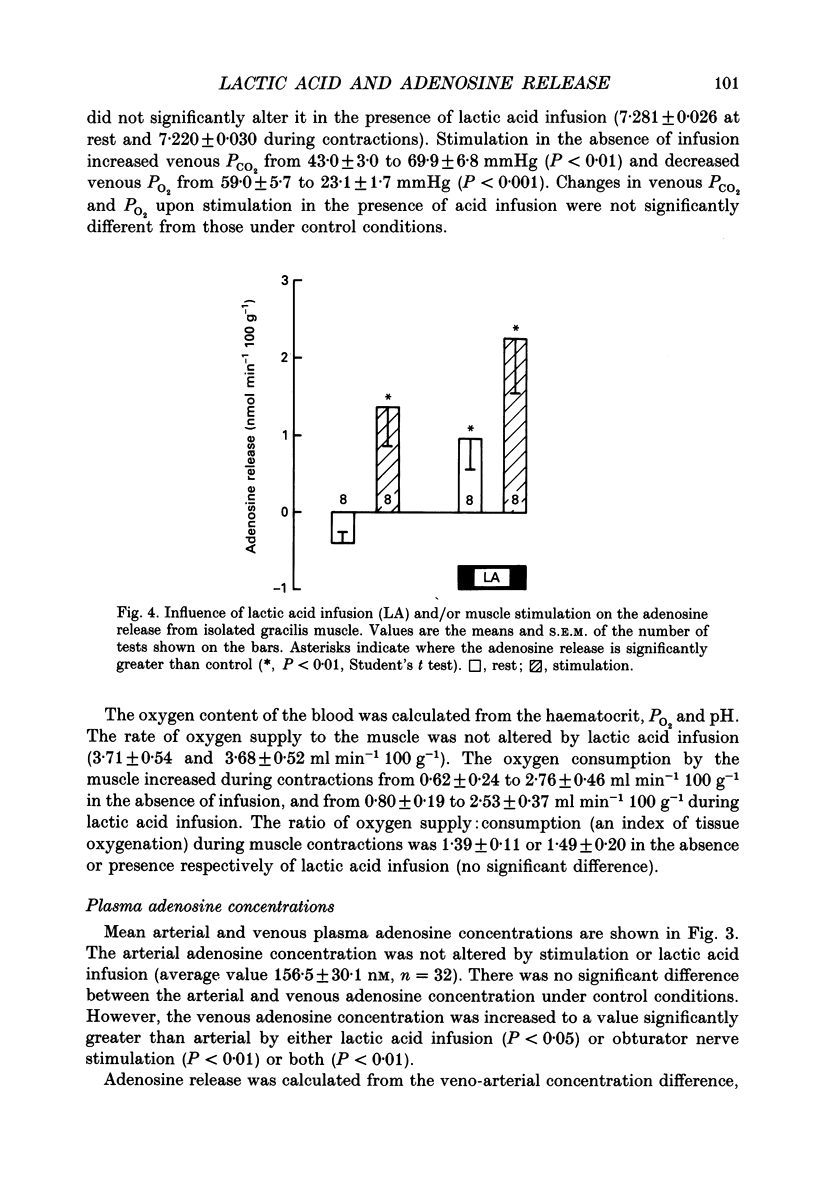
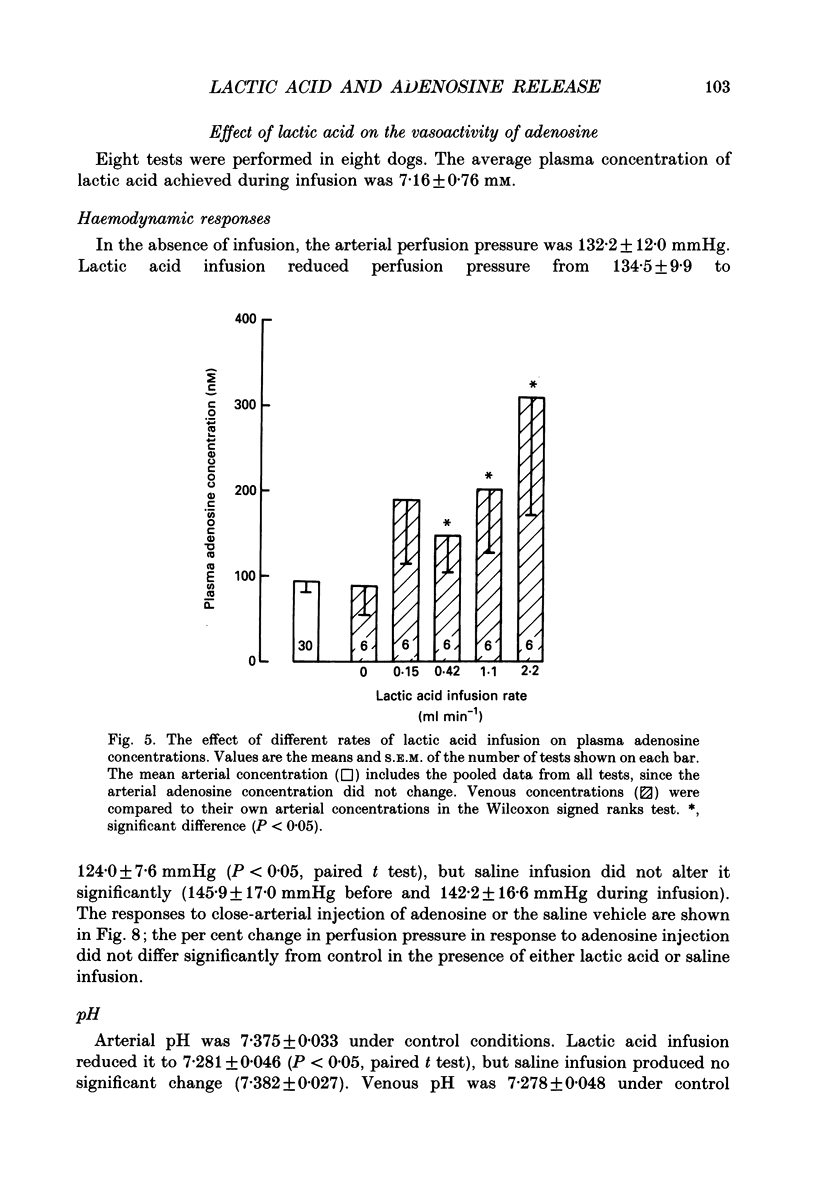
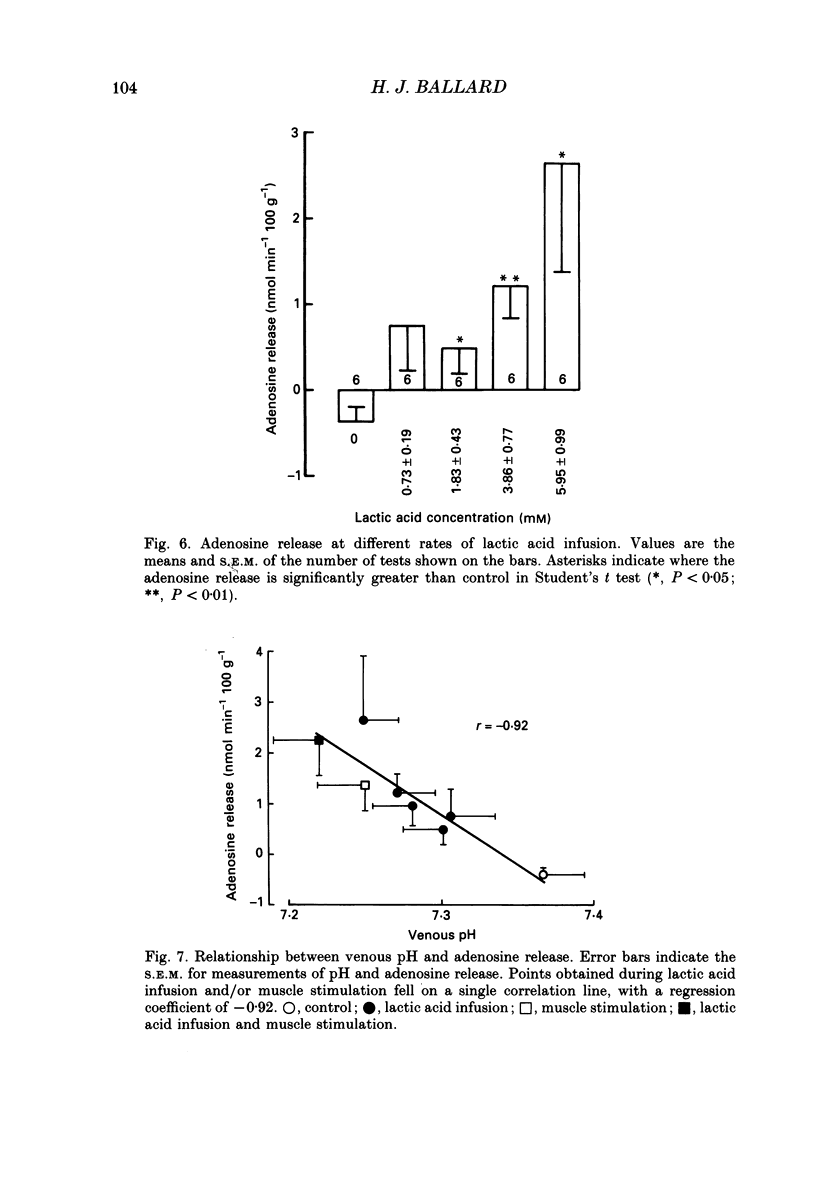
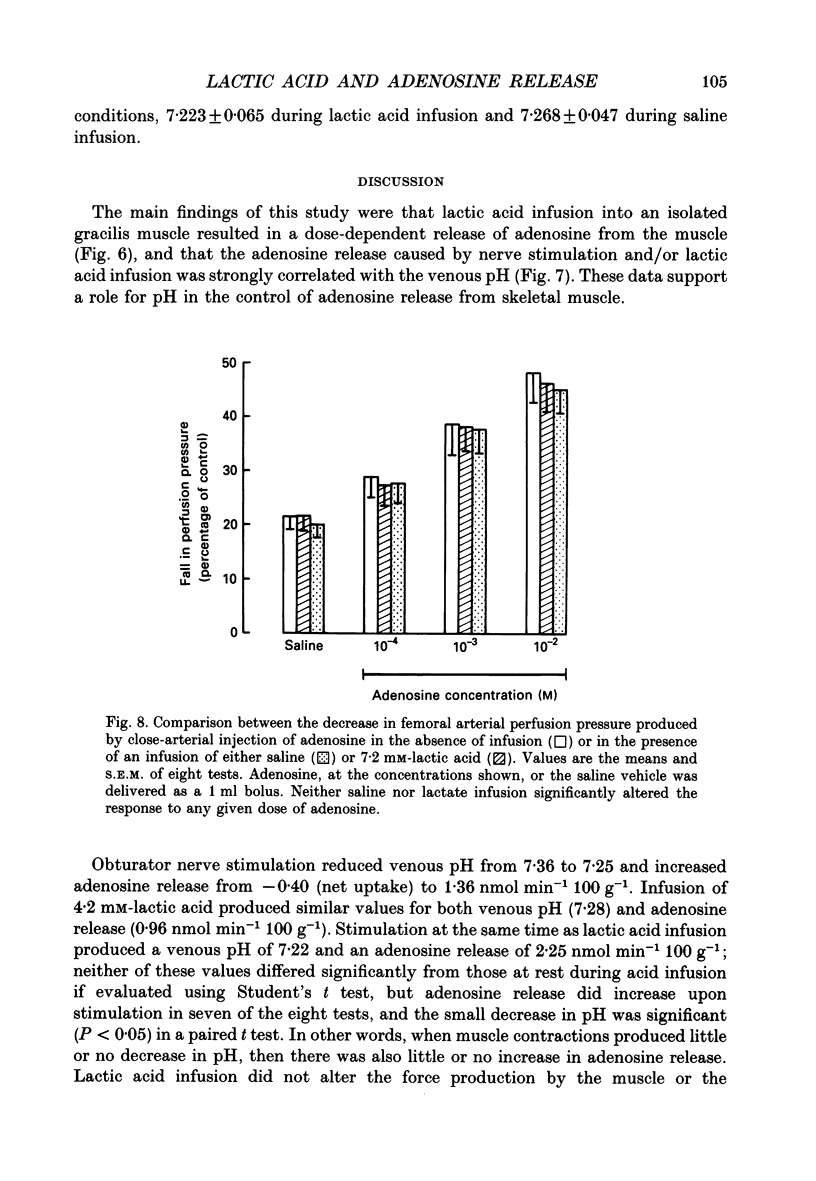
1. In anaesthetized and artificially ventilated dogs, a gracilis muscle was vascularly isolated and perfused at a constant flow rate of 11.9 +/- 2.2 ml min-1 100 g-1 (mean +/- S.E.M., n = 16; equivalent to 170.2 +/- 21.3% of its resting free flow). 2. Stimulation (3 Hz) of the obturator nerve produced twitch contractions of the gracilis muscle, reduced venous pH from 7.366 +/- 0.027 to 7.250 +/- 0.031 (n = 5), increased oxygen consumption from 0.62 +/- 0.24 to 2.76 +/- 0.46 ml min-1 100 g-1 (n = 5) and increased adenosine release from -0.40 +/- 0.14 (net uptake) to 1.36 +/- 0.50 nmol min-1 100 g-1 (n = 8). 3. Infusion of lactic acid (4.2 mM) into the artery reduced venous pH to 7.281 +/- 0.026 (n = 5) and increased adenosine release to 0.96 +/- 0.40 nmol min-1 100 g-1 (n = 8), but did not significantly alter oxygen consumption (0.80 +/- 0.19 ml min-1 100 g-1; n = 5). Stimulation (3 Hz) in the presence of lactic acid infusion produced no further significant changes in venous pH or adenosine release, but increased oxygen consumption to 2.53 +/- 0.37 ml min-1 100 g-1 (n = 5). 4. Infusion of a range of lactic acid concentrations (> or = 1.83 mM) produced dose-dependent increases in adenosine release. The maximum lactic acid concentration tested (5.95 mM) reduced venous pH to 7.249 +/- 0.023 (n = 5) and increased adenosine release to 2.64 +/- 1.26 nmol min-1 100 g-1 (n = 6). 5. A strong correlation existed between the adenosine release and the venous pH (r = -0.92); points obtained during muscle stimulation and/or lactic acid infusion fell on a single correlation line. 6. The vasoactivity of adenosine administered by close-arterial injection was unaltered by infusion of either lactic acid (7.2 mM) or saline. 7. These results suggest that the release of adenosine from skeletal muscle can be induced by a decrease in pH (probably at an intracellular site), and that this mechanism may contribute to the release of adenosine during muscle contractions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard H. J., Cotterrell D., Karim F. Analysis of submicromolar concentrations of adenosine in plasma using reversed phase high-performance liquid chromatography. J Pharm Biomed Anal. 1986;4(2):207–219. doi: 10.1016/0731-7085(86)80043-7. [DOI] [PubMed] [Google Scholar]
- Ballard H. J., Cotterrell D., Karim F. Appearance of adenosine in venous blood from the contracting gracilis muscle and its role in vasodilatation in the dog. J Physiol. 1987 Jun;387:401–413. doi: 10.1113/jphysiol.1987.sp016580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard H. J., Cotterrell D., Karim F. The influence of blood flow rate on adenosine release from contracting dog skeletal muscle. Q J Exp Physiol. 1989 Mar;74(2):97–107. doi: 10.1113/expphysiol.1989.sp003267. [DOI] [PubMed] [Google Scholar]
- Bardenheuer H., Whelton B., Sparks H. V., Jr Adenosine release by the isolated guinea pig heart in response to isoproterenol, acetylcholine, and acidosis: the minimal role of vascular endothelium. Circ Res. 1987 Oct;61(4):594–600. doi: 10.1161/01.res.61.4.594. [DOI] [PubMed] [Google Scholar]
- Belloni F. L., Bruttig S. P., Rubio R., Berne R. M. Uptake and release of adenosine by cultured rat aortic smooth muscle. Microvasc Res. 1986 Sep;32(2):200–210. doi: 10.1016/0026-2862(86)90054-3. [DOI] [PubMed] [Google Scholar]
- Belloni F. L., Phair R. D., Sparks H. V. The role of adenosine in prolonged vasodilation following flow-restricted exercise of canine skeletal muscle. Circ Res. 1979 Jun;44(6):759–766. doi: 10.1161/01.res.44.6.759. [DOI] [PubMed] [Google Scholar]
- Berne R. M., Rubio R., Dobson J. G., Jr, Curnish R. R. Adenosine and adenine nucleotides as possible mediators of cardiac and skeletal muscle blood flow regulation. Circ Res. 1971 Jan;28(Suppl):115+–115+. [PubMed] [Google Scholar]
- Bockman E. L., Berne R. M., Rubio R. Adenosine and active hyperemia in dog skeletal muscle. Am J Physiol. 1976 Jun;230(6):1531–1537. doi: 10.1152/ajplegacy.1976.230.6.1531. [DOI] [PubMed] [Google Scholar]
- Bockman E. L., Berne R. M., Rubio R. Release of adenosine and lack of release of ATP from contracting skeletal muscle. Pflugers Arch. 1975 Mar 26;355(3):229–241. doi: 10.1007/BF00583686. [DOI] [PubMed] [Google Scholar]
- Dobson J. G., Jr, Rubio R., Berne R. M. Role of adenine nucleotides, adenosine, and inorganic phosphate in the regulation of skeletal muscle blood flow. Circ Res. 1971 Oct;29(4):375–384. doi: 10.1161/01.res.29.4.375. [DOI] [PubMed] [Google Scholar]
- Downing S. E., Lee J. C., Weinstein E. M. Coronary dilator actions of adenosine and CO2 in experimental diabetes. Am J Physiol. 1982 Aug;243(2):H252–H258. doi: 10.1152/ajpheart.1982.243.2.H252. [DOI] [PubMed] [Google Scholar]
- Karim F., Ballard H. J., Cotterrell D. Changes in adenosine release and blood flow in the contracting dog gracilis muscle. Pflugers Arch. 1988 Jul;412(1-2):106–112. doi: 10.1007/BF00583738. [DOI] [PubMed] [Google Scholar]
- Kille J. M., Klabunde R. E. Adenosine as a mediator of postcontraction hyperemia in dog gracilis muscle. Am J Physiol. 1984 Feb;246(2 Pt 2):H274–H282. doi: 10.1152/ajpheart.1984.246.2.H274. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Mainwood G. W., Thoden J. S. The influence of extracellular buffer concentration and propionate on lactate efflux from frog muscle. Pflugers Arch. 1986 May;406(5):472–479. doi: 10.1007/BF00583369. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Thomas R. C. A microelectrode study of the mechanisms of L-lactate entry into and release from frog sartorius muscle. J Physiol. 1988 Jun;400:459–479. doi: 10.1113/jphysiol.1988.sp017132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall R. J. The influence of acid base equilibrium on the activities of blood vessels. J Physiol. 1928 Mar 30;65(1):25–32. doi: 10.1113/jphysiol.1928.sp002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S. J., Ghai G. Effect of adenosine on the relaxation of coronary arteries at varying pH values. Basic Res Cardiol. 1981 Jul-Aug;76(4):380–386. doi: 10.1007/BF01908327. [DOI] [PubMed] [Google Scholar]
- Mustafa S. J., Mansour M. M. Effect of perfusate pH on coronary flow and adenosine release in isolated rabbit heart. Proc Soc Exp Biol Med. 1984 May;176(1):22–26. doi: 10.3181/00379727-176-41836. [DOI] [PubMed] [Google Scholar]
- Phair R. D., Sparks H. V. Adenosine content of skeletal muscle during active hyperemia and ischemic contraction. Am J Physiol. 1979 Jul;237(1):H1–H9. doi: 10.1152/ajpheart.1979.237.1.H1. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of D- and L-lactate and of DMO in rat diaphragm. J Physiol. 1975 Jul;249(1):1–25. doi: 10.1113/jphysiol.1975.sp011000. [DOI] [PMC free article] [PubMed] [Google Scholar]


