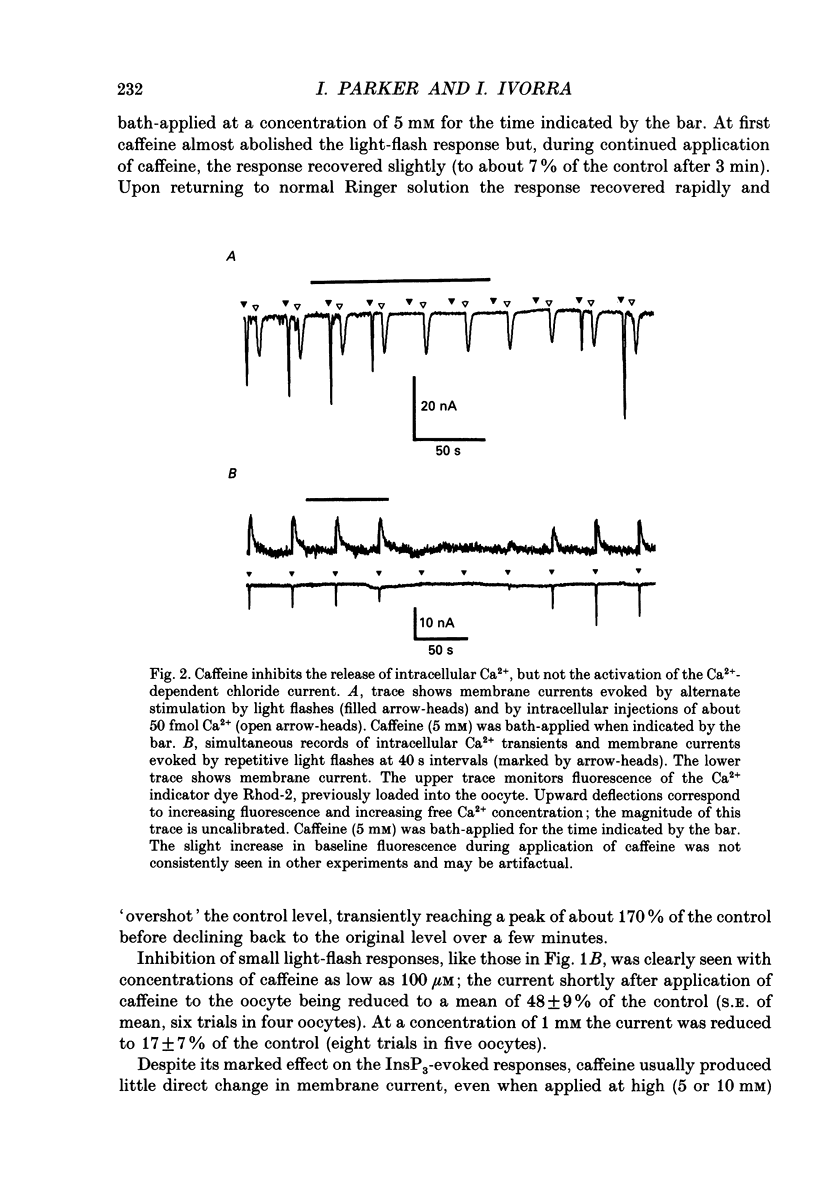
Abstract
1. Voltage-clamp recording of Ca(2+)-activated chloride currents in Xenopus oocytes was used to study the effects of caffeine on the liberation of intracellular Ca2+ induced by photo-release of inositol 1,4,5-trisphosphate (InsP3) from caged InsP3. Bath application of caffeine, at concentrations between 0.1 and 10 mM, reduced or abolished the current evoked by photo-release of InsP3 and by microinjection of InsP3. 2. Caffeine did not appreciably reduce currents evoked by injection of Ca2+ into oocytes, whereas measurements using the Ca2+ indicator Rhod-2 showed that it instead inhibited the liberation of Ca2+ by InsP3. 3. Caffeine increased the threshold amount of InsP3 required to evoke a current response and proportionally reduced the currents evoked by suprathreshold levels of InsP3. 4. Theophylline and 3-isobutyl-1-methylxanthine (IBMX) were much less potent than caffeine, and few changes were seen in the InsP3 responses following application of forskolin or intracellular injection of cyclic AMP. Thus, inhibition of InsP3 responses by caffeine does not arise through inhibition of phosphodiesterase enzymes. 5. Even at high (10 mM) concentrations, caffeine did not itself elicit any clear Ca(2+)-activated current. It is therefore unlikely that inhibition of the InsP3 responses arise because caffeine itself liberates Ca2+ from intracellular stores. 6. The site of action of caffeine is intracellular, because injections of caffeine into the oocyte strongly inhibited responses to InsP3, whereas local extracellular applications of similar amounts were almost without effect.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Hill T. D., Berggren P. O., Boynton A. L. Heparin inhibits inositol trisphosphate-induced calcium release from permeabilized rat liver cells. Biochem Biophys Res Commun. 1987 Dec 31;149(3):897–901. doi: 10.1016/0006-291x(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Campos-Gonzalez R., Kindmark H., Boynton A. L. Inhibition of inositol trisphosphate-stimulated calcium mobilization by calmodulin antagonists in rat liver epithelial cells. J Biol Chem. 1988 Nov 5;263(31):16479–16484. [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989 Nov;9(11):4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Seiler S. M., Arnold A. J., Stanton H. C. Inhibitors of inositol trisphosphate-induced Ca2+ release from isolated platelet membrane vesicles. Biochem Pharmacol. 1987 Oct 15;36(20):3331–3337. doi: 10.1016/0006-2952(87)90307-8. [DOI] [PubMed] [Google Scholar]
- Shah J., Pant H. C. Potassium-channel blockers inhibit inositol trisphosphate-induced calcium release in the microsomal fractions isolated from the rat brain. Biochem J. 1988 Mar 1;250(2):617–620. doi: 10.1042/bj2500617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]


