Abstract
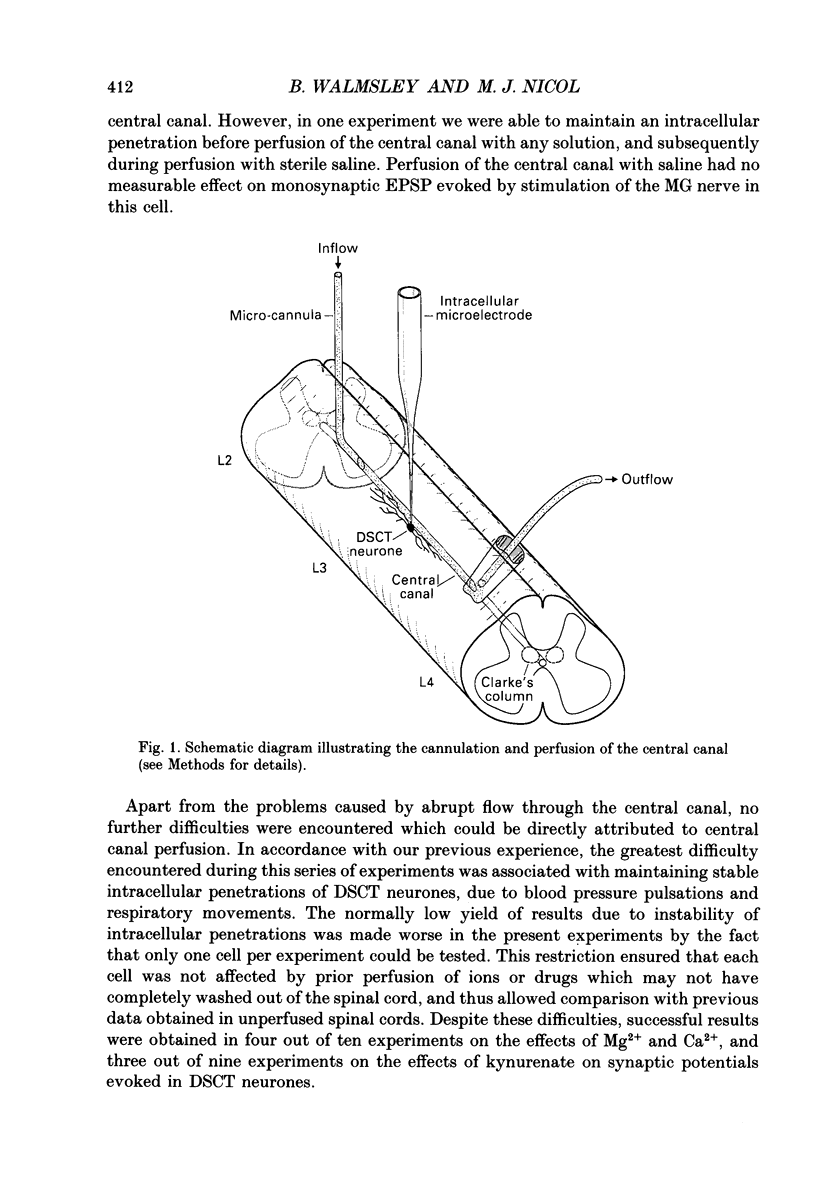
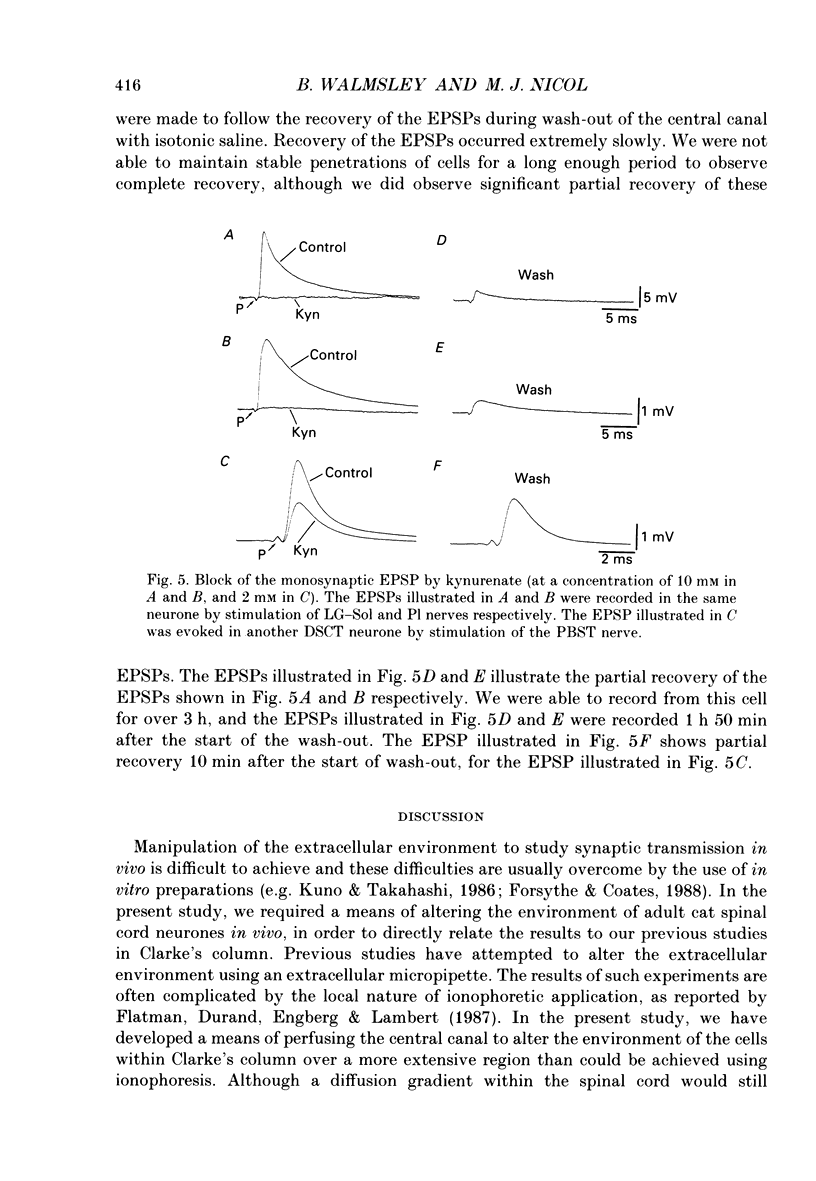
1. A technique was developed for perfusing the central canal of the cat spinal cord over a defined region to alter the extracellular environment and examine the effects of various ions and pharmacological agents on synaptic transmission in vivo. 2. Monosynaptic excitatory postsynaptic potentials (EPSPs) evoked by hindlimb muscle nerve stimulation were recorded intracellularly from dorsal spinocerebellar tract (DSCT) neurones in Clarke's column, in close proximity to the central canal. 3. The effects of central canal perfusion of solutions containing Ca2+, Mg2+ and kynurenate on the monosynaptic afferent EPSP were examined. 4. Perfusion of the central canal with solutions containing a high Mg2+ concentration completely and reversibly blocked the monosynaptic EPSP, while perfusion with solutions containing a high Ca2+ concentration produced up to a fourfold increase in the peak amplitude of the EPSP. This large increase in the EPSP indicates that the pool of quanta available for release is considerably greater than estimated from previous quantal analysis studies at this synaptic connection. 5. Perfusion of the central canal with kynurenate, an antagonist at excitatory amino acid receptors, resulted in a complete block of the monosynaptic EPSP in DSCT neurones. This provides direct evidence that an excitatory amino acid, such as glutamate, is released from primary muscle afferent terminals in Clarke's column of the cat spinal cord in vivo.
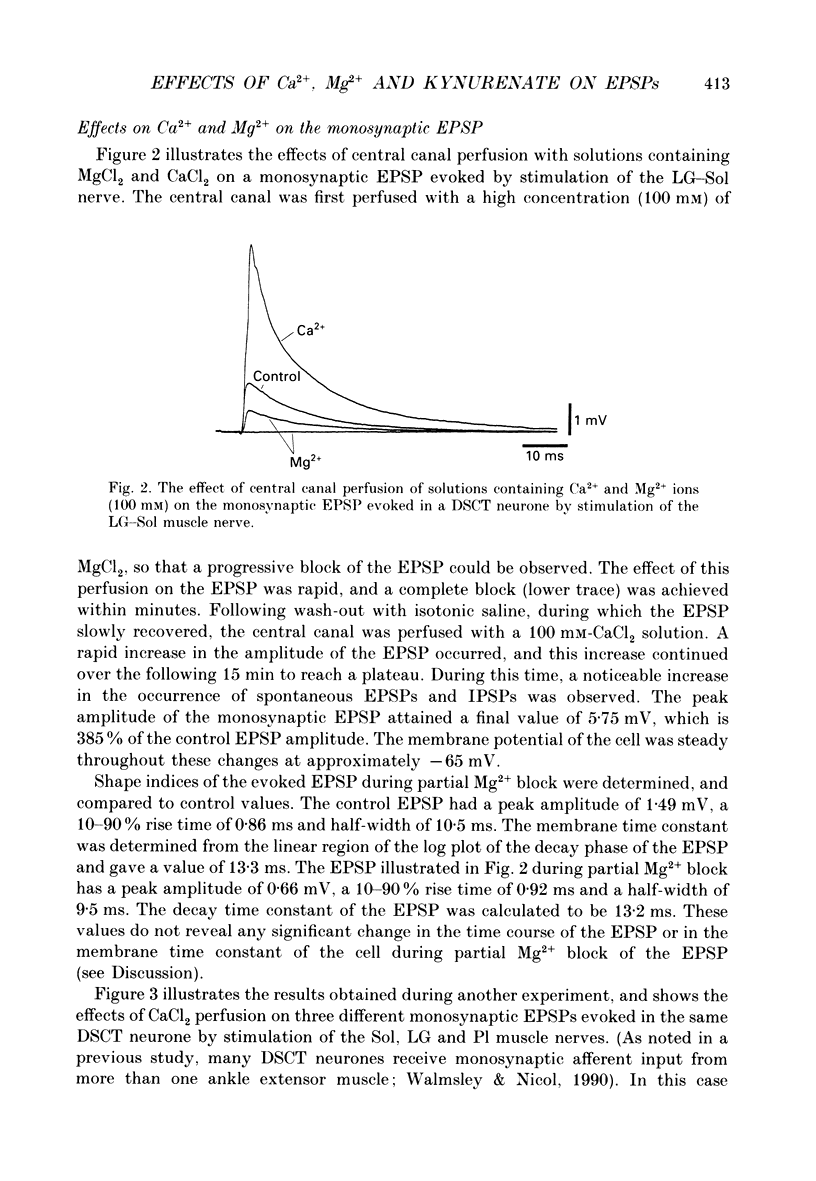
Full text
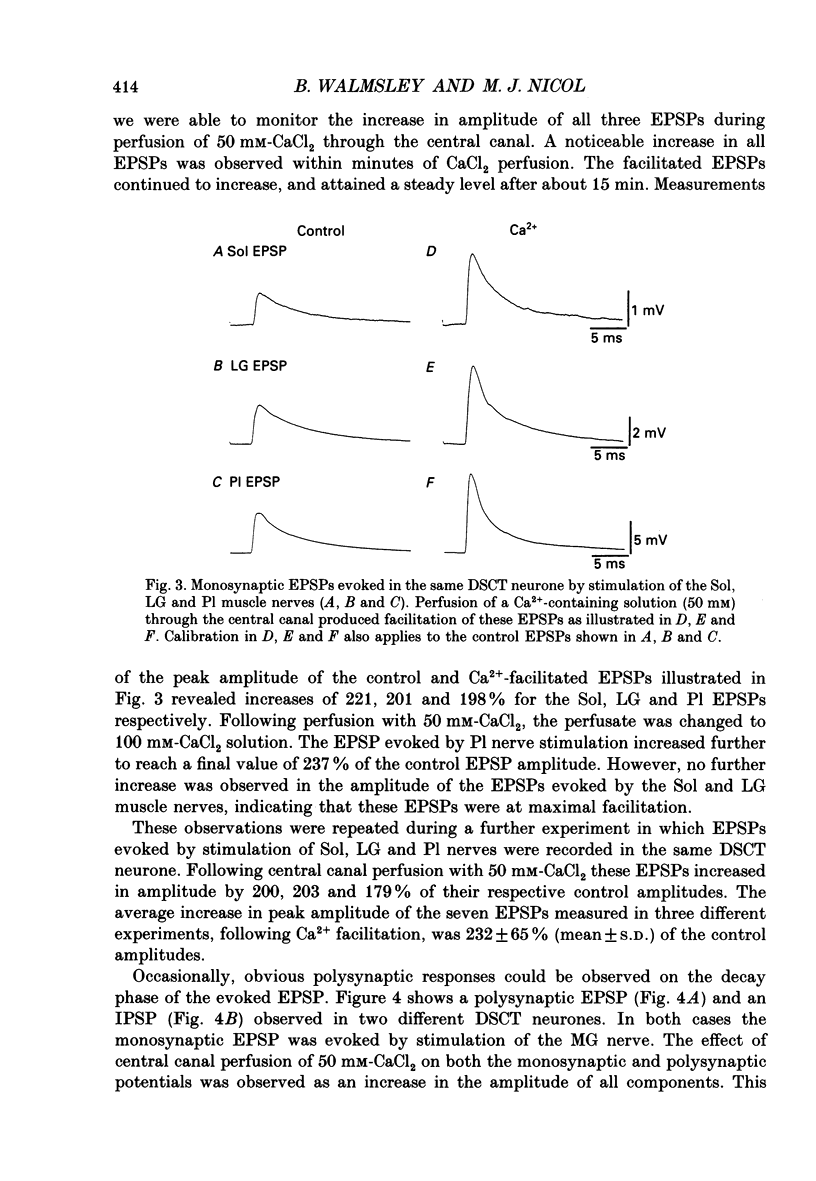
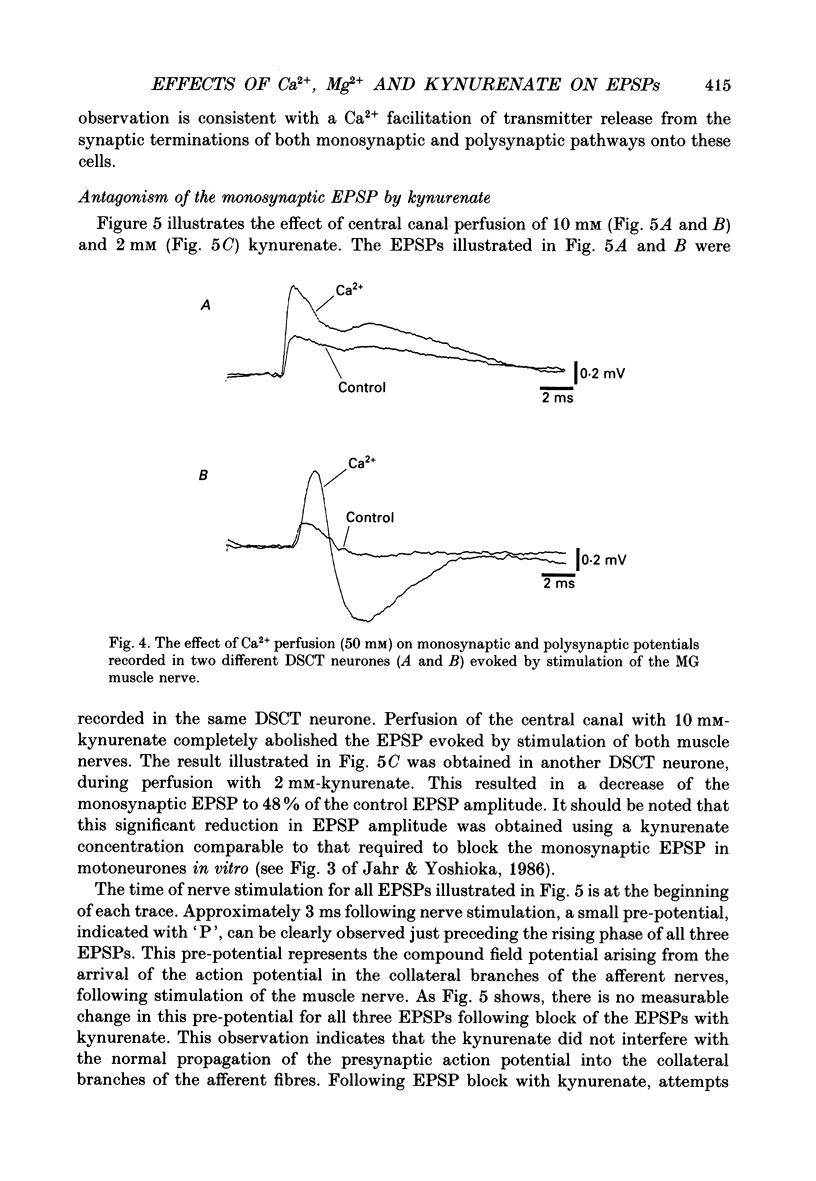
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies J., Watkins J. C. Depressant actions of gamma-D-glutamylaminomethyl sulfonate (GAMS) on amino acid-induced and synaptic excitation in the cat spinal cord. Brain Res. 1985 Feb 18;327(1-2):113–120. doi: 10.1016/0006-8993(85)91505-7. [DOI] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Properties of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):125–144. doi: 10.1111/j.1748-1716.1969.tb04558.x. [DOI] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Unitary components in the activation of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):145–158. doi: 10.1111/j.1748-1716.1969.tb04559.x. [DOI] [PubMed] [Google Scholar]
- Evans R. H. The pharmacology of segmental transmission in the spinal cord. Prog Neurobiol. 1989;33(4):255–279. doi: 10.1016/0301-0082(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Coates R. T. A chamber for electrophysiological recording from cultured neurones allowing perfusion and temperature control. J Neurosci Methods. 1988 Aug;25(1):19–27. doi: 10.1016/0165-0270(88)90116-1. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong A. H., Lanthorn T. H., Cotman C. W. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983 Aug 22;273(1):170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- Houchin J., Maxwell D. J., Fyffe R. E., Brown A. G. Light and electron microscopy of dorsal spinocerebellar tract neurones in the cat: an intracellular horseradish peroxidase study. Q J Exp Physiol. 1983 Oct;68(4):719–732. doi: 10.1113/expphysiol.1983.sp002761. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. Synaptic transmission between dorsal root ganglion and dorsal horn neurons in culture: antagonism of monosynaptic excitatory postsynaptic potentials and glutamate excitation by kynurenate. J Neurosci. 1985 Aug;5(8):2281–2289. doi: 10.1523/JNEUROSCI.05-08-02281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986 Jan;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Factors responsible for multiple discharge of neurons in Clarke's column. J Neurophysiol. 1968 Jul;31(4):624–638. doi: 10.1152/jn.1968.31.4.624. [DOI] [PubMed] [Google Scholar]
- Kuno M., Takahashi T. Effects of calcium and magnesium on transmitter release at Ia synapses of rat spinal motoneurones in vitro. J Physiol. 1986 Jul;376:543–553. doi: 10.1113/jphysiol.1986.sp016169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Randić M., Miletić V., Loewy A. D. A morphological study of cat dorsal spinocerebellar tract neurons after intracellular injection of horseradish peroxidase. J Comp Neurol. 1981 May 20;198(3):453–466. doi: 10.1002/cne.901980306. [DOI] [PubMed] [Google Scholar]
- Robinson M. B., Anderson K. D., Koerner J. F. Kynurenic acid as an antagonist of hippocampal excitatory transmission. Brain Res. 1984 Aug 20;309(1):119–126. doi: 10.1016/0006-8993(84)91015-1. [DOI] [PubMed] [Google Scholar]
- Tracey D. J., Walmsley B. Synaptic input from identified muscle afferents to neurones of the dorsal spinocerebellar tract in the cat. J Physiol. 1984 May;350:599–614. doi: 10.1113/jphysiol.1984.sp015220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Edwards F. R., Tracey D. J. Nonuniform release probabilities underlie quantal synaptic transmission at a mammalian excitatory central synapse. J Neurophysiol. 1988 Sep;60(3):889–908. doi: 10.1152/jn.1988.60.3.889. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Edwards F. R., Tracey D. J. The probabilistic nature of synaptic transmission at a mammalian excitatory central synapse. J Neurosci. 1987 Apr;7(4):1037–1046. doi: 10.1523/JNEUROSCI.07-04-01037.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Nicol M. J. Location and morphology of dorsal spinocerebellar tract neurons that receive monosynaptic afferent input from ankle extensor muscles in cat hindlimb. J Neurophysiol. 1990 Feb;63(2):286–293. doi: 10.1152/jn.1990.63.2.286. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Stuklis R. Effects of spatial and temporal dispersion of synaptic input on the time course of synaptic potentials. J Neurophysiol. 1989 Apr;61(4):681–687. doi: 10.1152/jn.1989.61.4.681. [DOI] [PubMed] [Google Scholar]
- Walmsley B. Synaptic potentials evoked in cat dorsal spinocerebellar tract neurones by impulses in single group I muscle afferents. J Physiol. 1989 Aug;415:423–431. doi: 10.1113/jphysiol.1989.sp017729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Wieniawa-Narkiewicz E., Nicol M. J. The ultrastructural basis for synaptic transmission between primary muscle afferents and neurons in Clarke's column of the cat. J Neurosci. 1985 Aug;5(8):2095–2106. doi: 10.1523/JNEUROSCI.05-08-02095.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Wieniawa-Narkiewicz E., Nicol M. J. Ultrastructural evidence related to presynaptic inhibition of primary muscle afferents in Clarke's column of the cat. J Neurosci. 1987 Jan;7(1):236–243. doi: 10.1523/JNEUROSCI.07-01-00236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


