Abstract
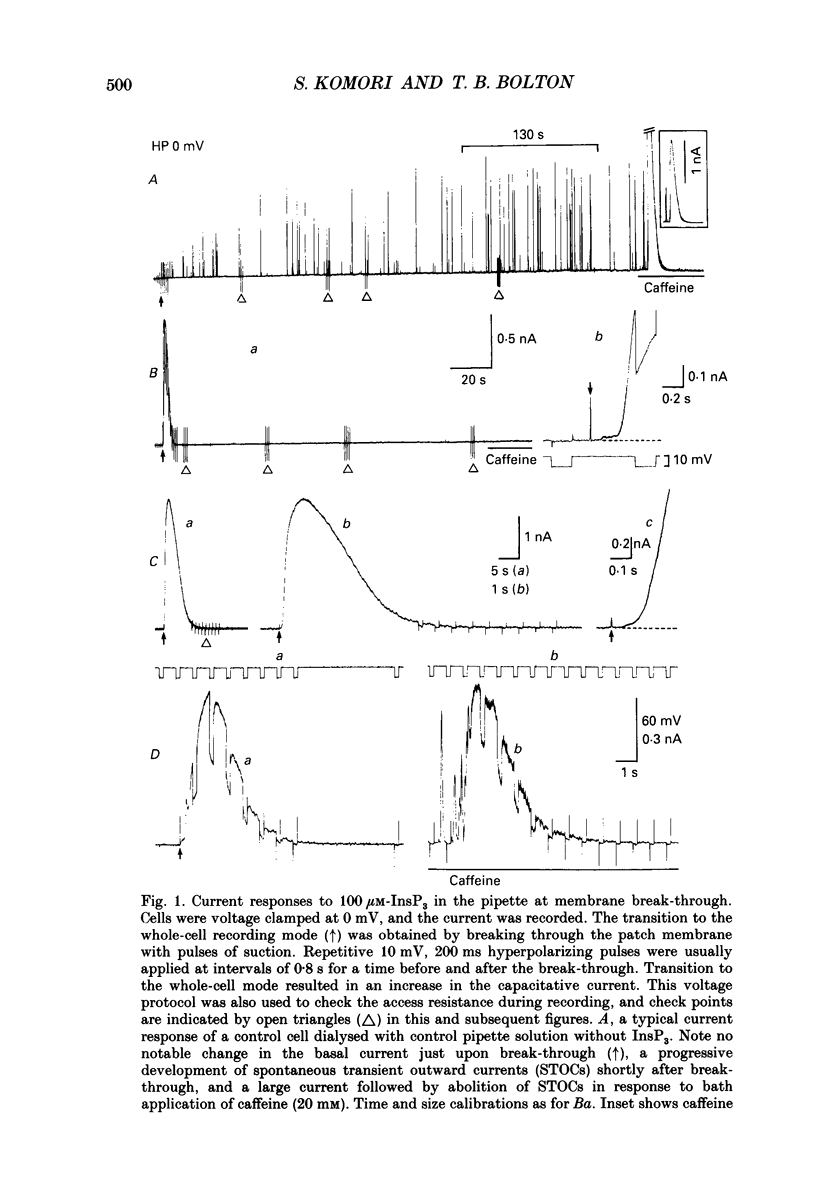
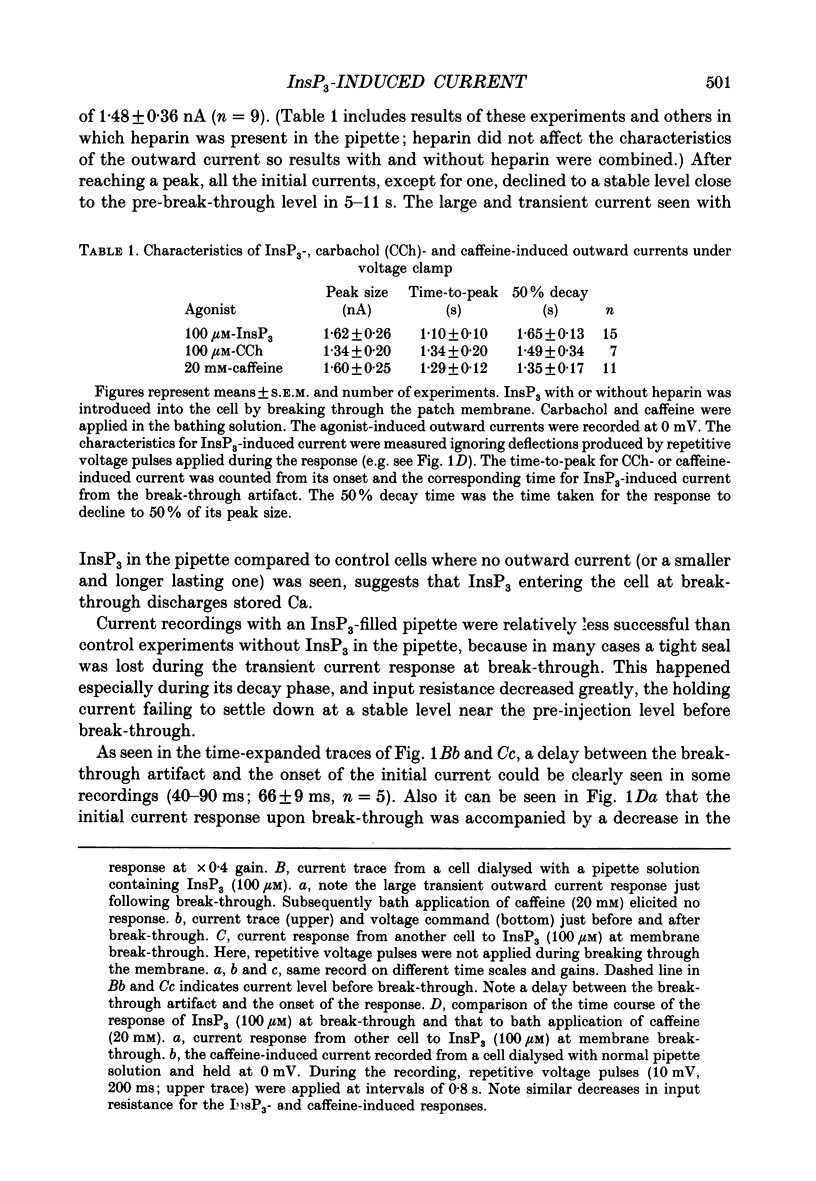
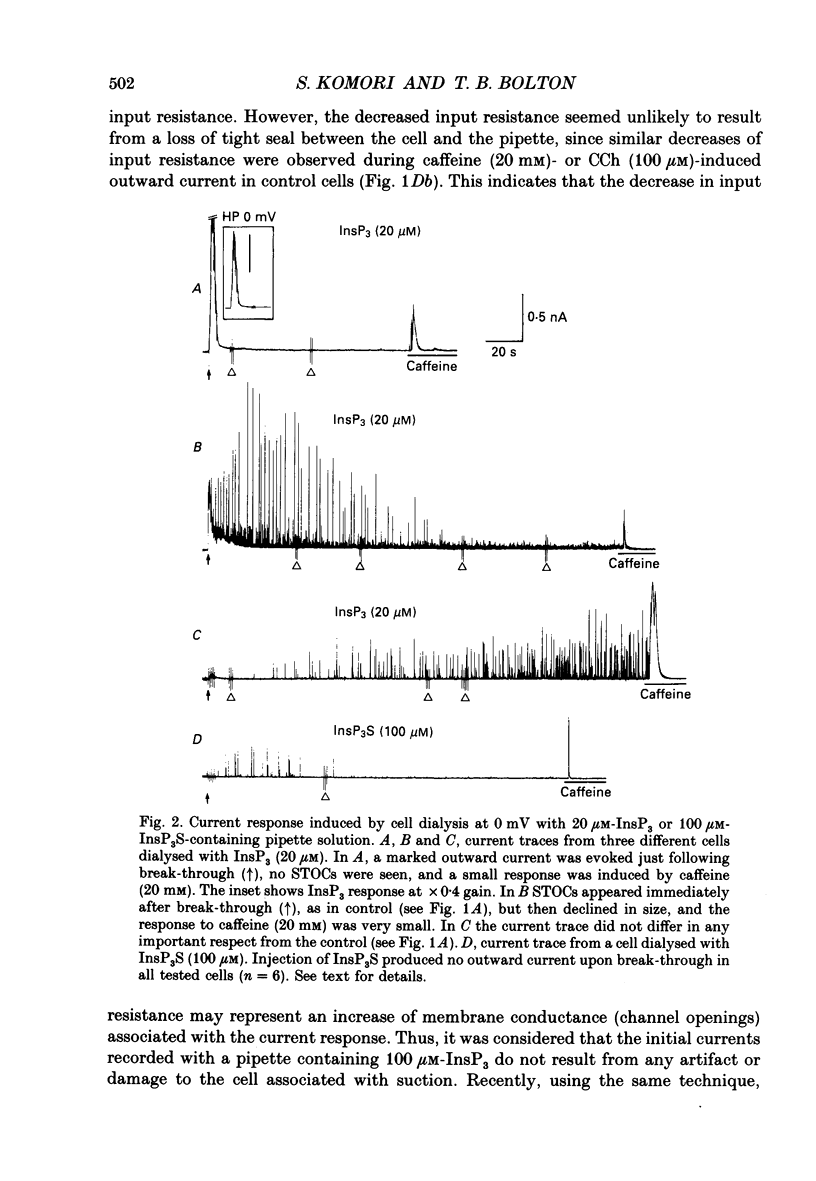
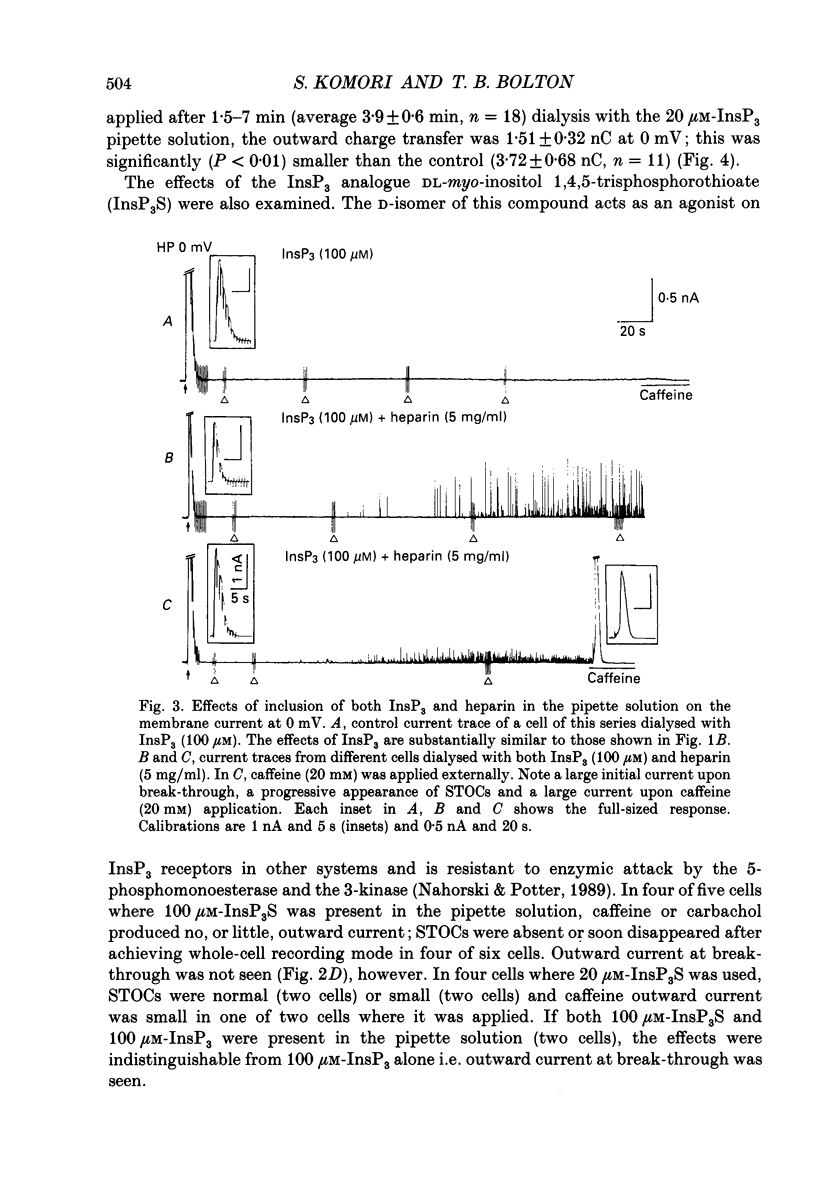
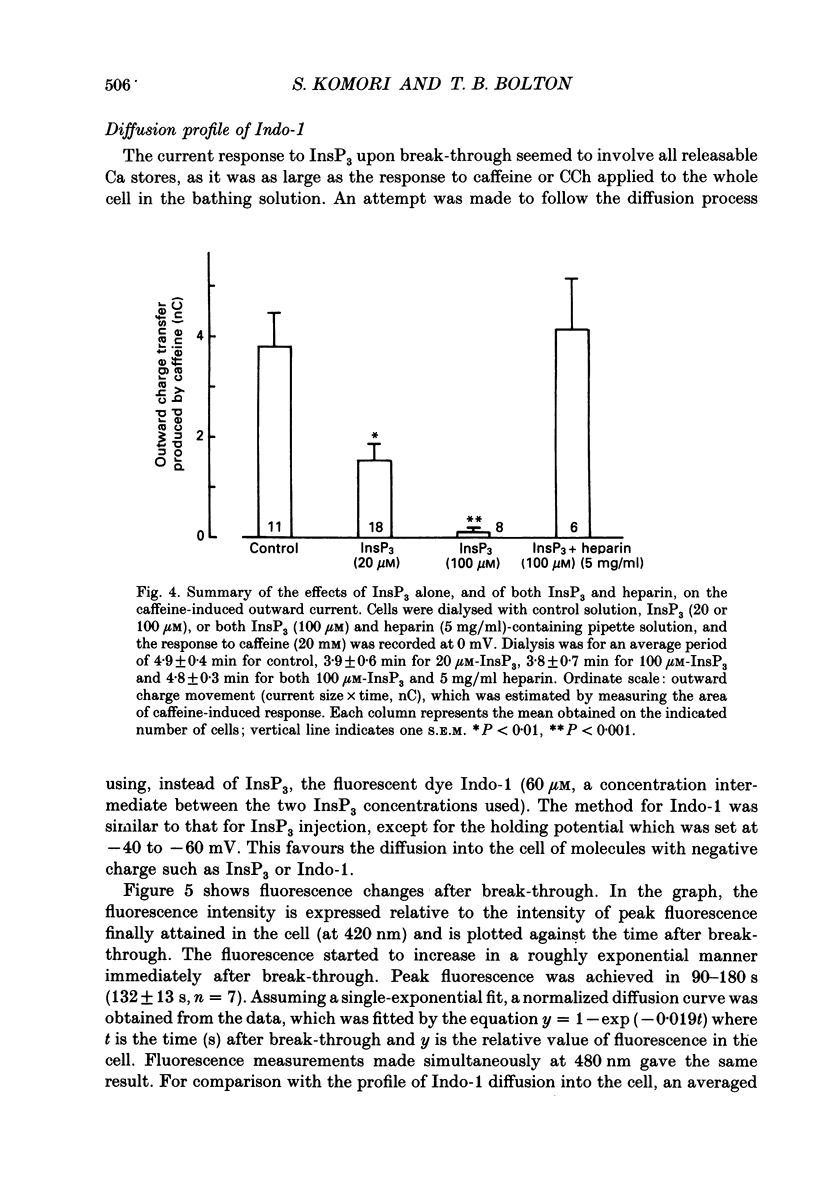
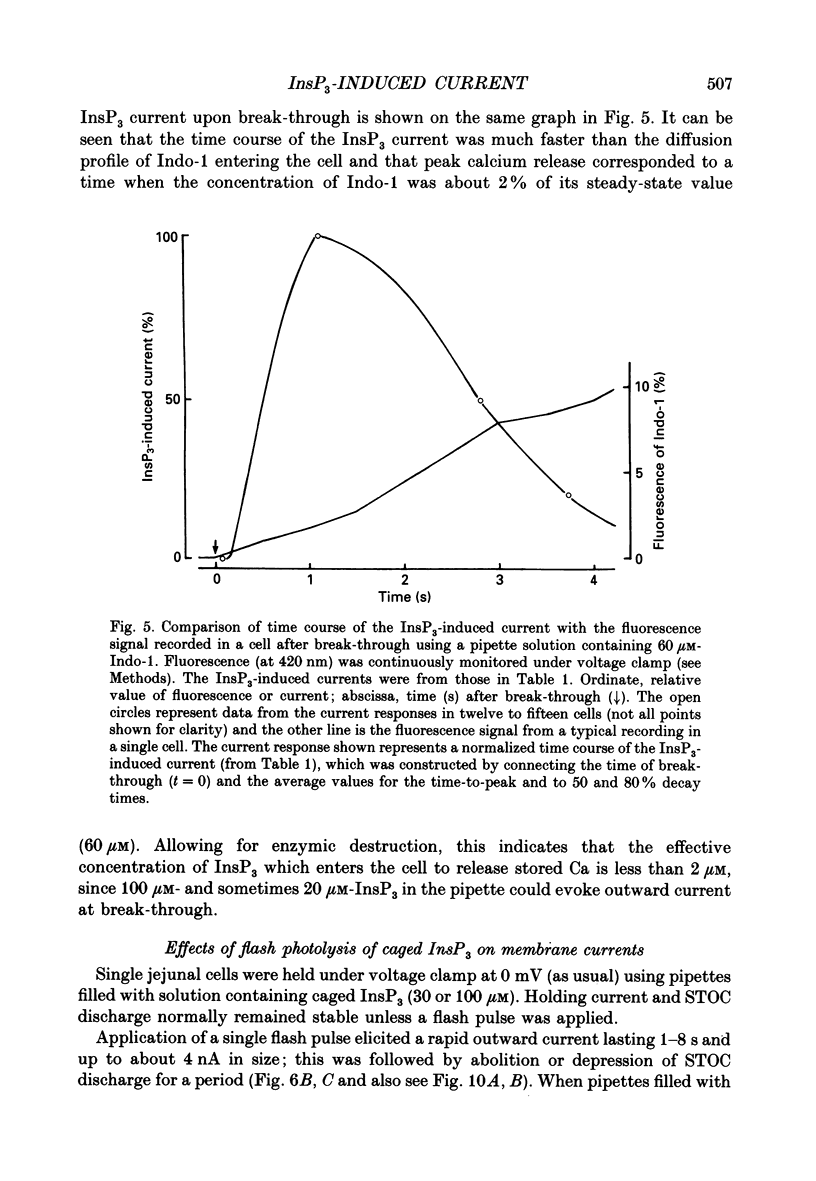
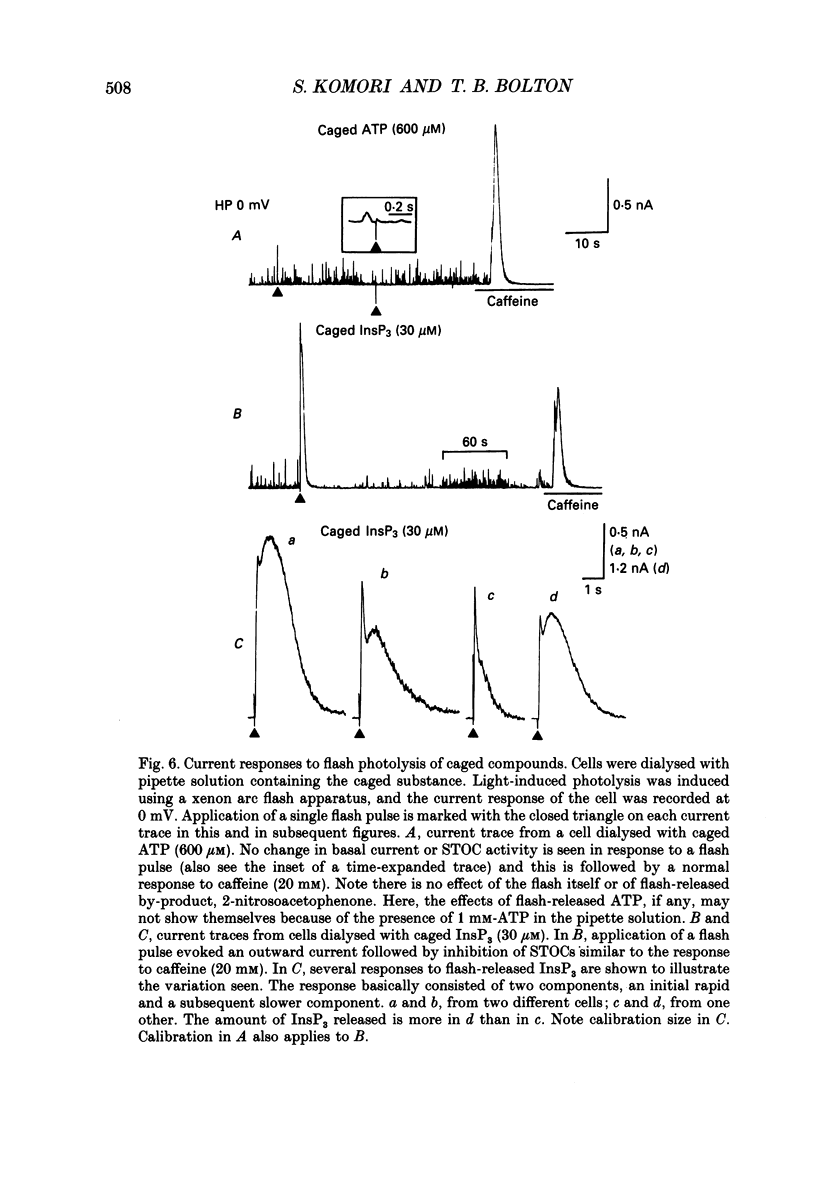
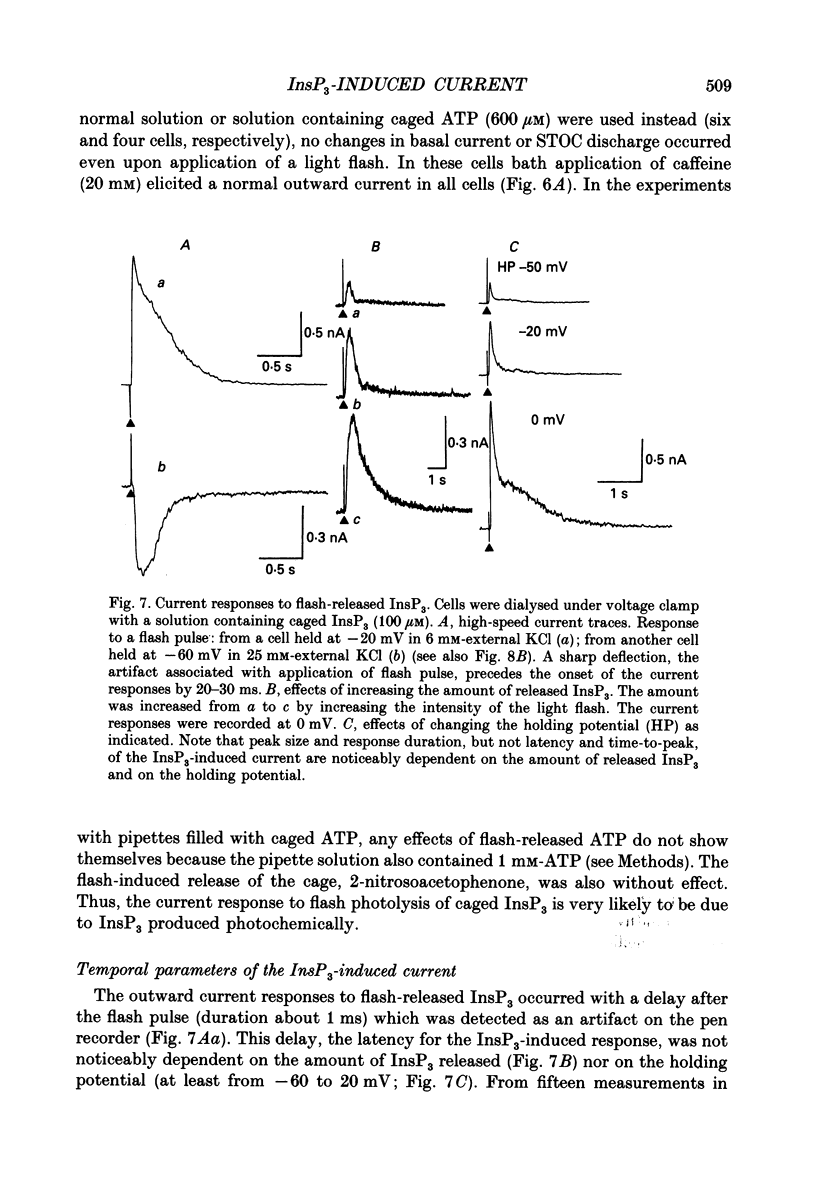
1. Single smooth muscle cells were isolated by enzymic digestion from the longitudinal muscle layer of rabbit jejunum, and the response of the cells to calcium (Ca2+) release by InsP3 (D-myo-inositol 1,4,5-trisphosphate) was studied. Changes in internal Ca2+ concentration were monitored by measuring Ca(2+)-activated K+ currents (outward currents) using the whole-cell voltage-clamp technique. 2. At break-through from cell-attached patch to whole-cell recording mode using a 100 microM-InsP3-filled pipette, cells exhibited a brief outward current which reached its peak in 1.1 s and terminated within 10 s. Following this the generation of spontaneous transient outward currents (STOCs) was inhibited. (STOCs are considered to represent bursts of openings of Ca(2+)-activated K+ channels in response to spontaneous discharges of Ca2+ from the stores.) When a pipette filled with 20 microM-InsP3 was used, similar current responses were also evoked, but some cells failed to respond. 3. The InsP3-induced outward current at membrane break-through was similar in size and time course to the outward current response of normal cells to bath-applied carbachol (CCh, 100 microM) or caffeine (20 mM). 4. Dialysis with InsP3-containing solution inhibited the caffeine-induced outward current, depending on the pipette InsP3 concentration. Inclusion of heparin (5 mg/ml) in the pipette completely prevented inhibition by InsP3 of the caffeine response and of STOC discharge. However, the InsP3-induced current at break-through remained unchanged, probably because of the slower rate of diffusion of heparin. 5. In cells dialysed with pipette solution containing 30 or 100 microM-caged InsP3, flash photolysis (producing up to 1.5 microM-InsP3) induced an outward current response after a latency of 31.0 +/- 1.8 ms (n = 15), which was followed by inhibition of STOCs. The reversal potential of the current to flash-release of InsP3 followed closely the Nernst potential for K+ ions (EK), suggesting negligible contributions from channels other than Ca(2+)-activated K+ channels. 6. Photolysis of caged InsP3 (30 or 100 microM) still produced a current response after 3-6 min in Ca(2+)-free (3 mM-EGTA added) bathing solution, but no response occurred if the cell was exposed to either caffeine (20 mM) or CCh (100 microM) to deplete Ca stores.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








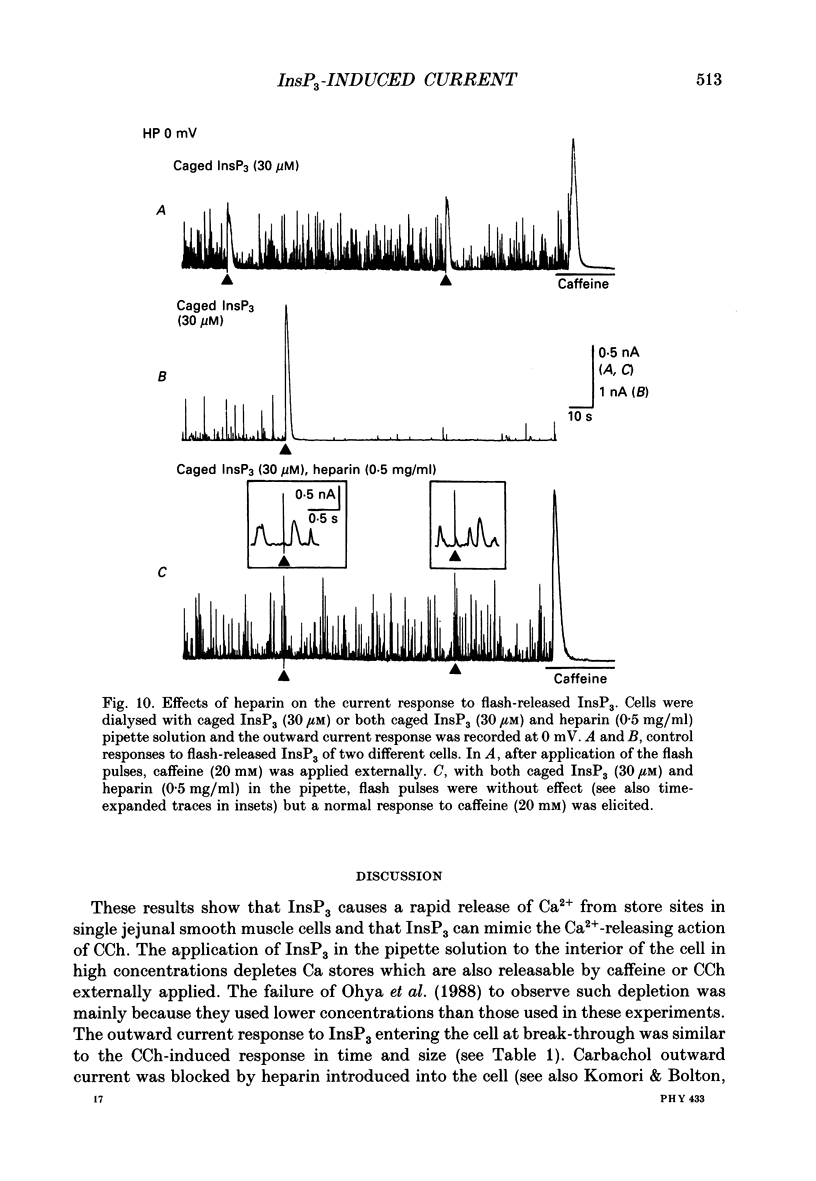




Selected References
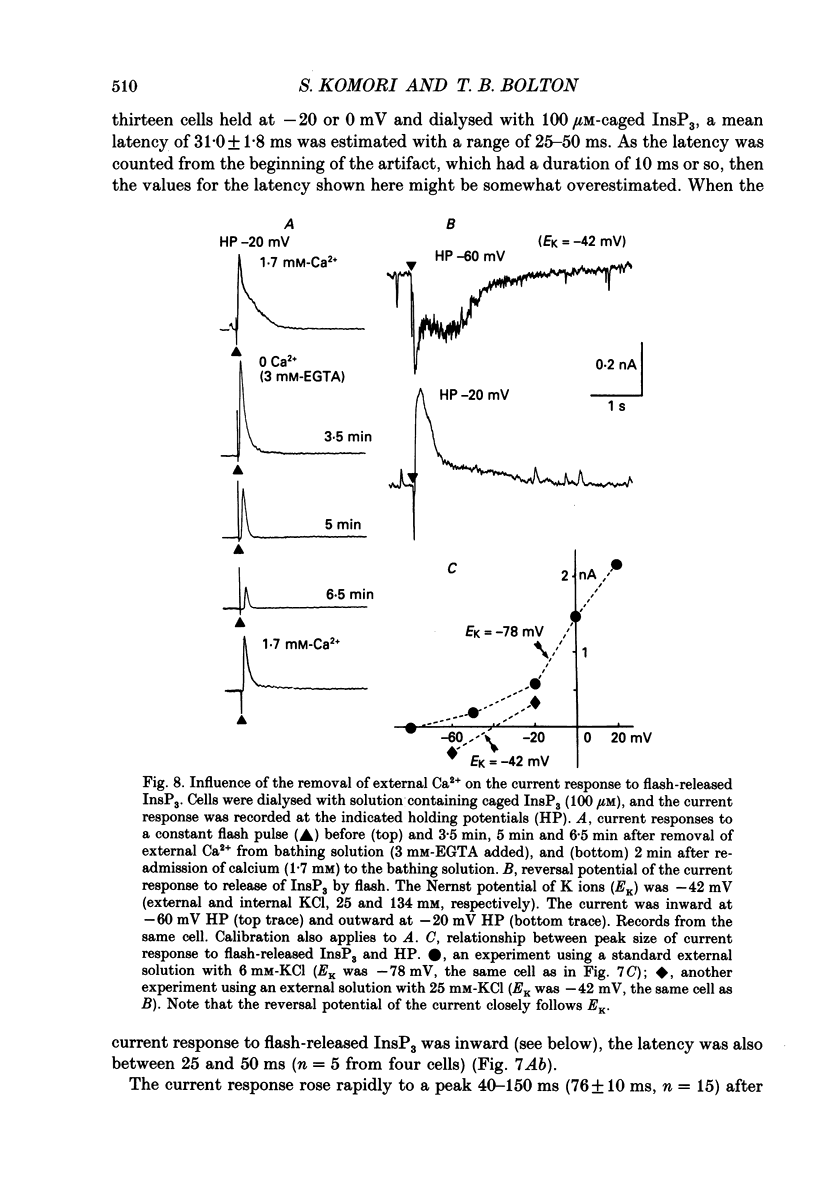
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Berggren P. O., Boynton A. L. Heparin inhibits inositol trisphosphate-induced calcium release from permeabilized rat liver cells. Biochem Biophys Res Commun. 1987 Dec 31;149(3):897–901. doi: 10.1016/0006-291x(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Somlyo A. V., Somlyo A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not the independent, calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem Biophys Res Commun. 1988 Jun 16;153(2):625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Actions of guanine nucleotides and cyclic nucleotides on calcium stores in single patch-clamped smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1989 Jul;97(3):973–982. doi: 10.1111/j.1476-5381.1989.tb12039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. P., Bolton T. B. A calcium-dependent rather than a G-protein mechanism is involved in the inward current evoked by muscarinic receptor stimulation in dialysed single smooth muscle cells of small intestine. Br J Pharmacol. 1988 Oct;95(2):325–327. doi: 10.1111/j.1476-5381.1988.tb11649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J Physiol. 1989 Dec;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. Activation and desensitization mechanisms of muscarinic current response in single pancreatic acinar cells of rats. J Physiol. 1989 Oct;417:343–359. doi: 10.1113/jphysiol.1989.sp017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski S. R., Potter B. V. Molecular recognition of inositol polyphosphates by intracellular receptors and metabolic enzymes. Trends Pharmacol Sci. 1989 Apr;10(4):139–144. doi: 10.1016/0165-6147(89)90165-x. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Yamaguchi K., Inoue R., Okabe K., Kitamura K., Hirata M., Kuriyama H. Effects of inositol phosphates on the membrane activity of smooth muscle cells of the rabbit portal vein. Pflugers Arch. 1988 Sep;412(4):382–389. doi: 10.1007/BF01907556. [DOI] [PubMed] [Google Scholar]
- Prestwich S. A., Bolton T. B. Mass determination of inositol phosphates: a comparison with radiotracer studies. Biochem Soc Trans. 1990 Aug;18(4):623–624. doi: 10.1042/bst0180623. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Walker J. W., Goldman Y. E., Trentham D. R., Kobayashi S., Kitazawa T., Somlyo A. V. Inositol trisphosphate, calcium and muscle contraction. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):399–414. doi: 10.1098/rstb.1988.0084. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Feeney J., Trentham D. R. Photolabile precursors of inositol phosphates. Preparation and properties of 1-(2-nitrophenyl)ethyl esters of myo-inositol 1,4,5-trisphosphate. Biochemistry. 1989 Apr 18;28(8):3272–3280. doi: 10.1021/bi00434a023. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Somlyo A. V., Goldman Y. E., Somlyo A. P., Trentham D. R. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987 May 21;327(6119):249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]


