Abstract
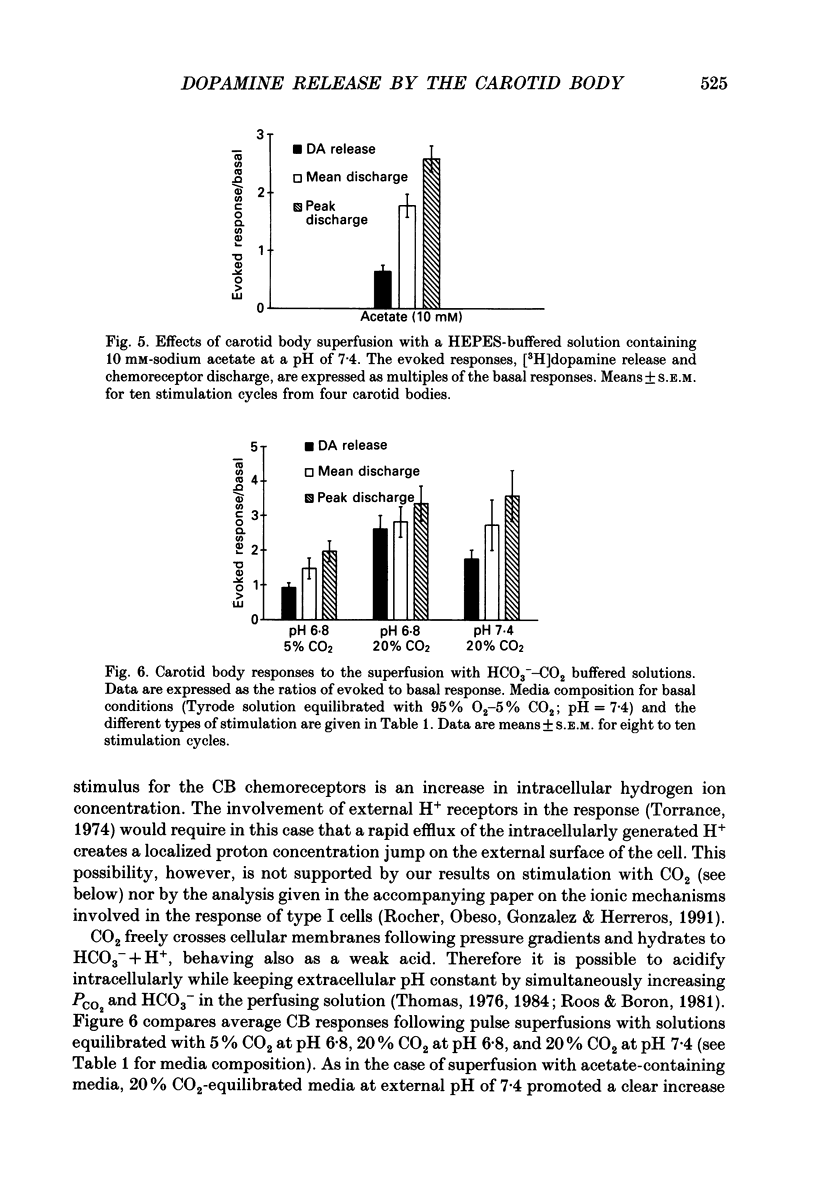
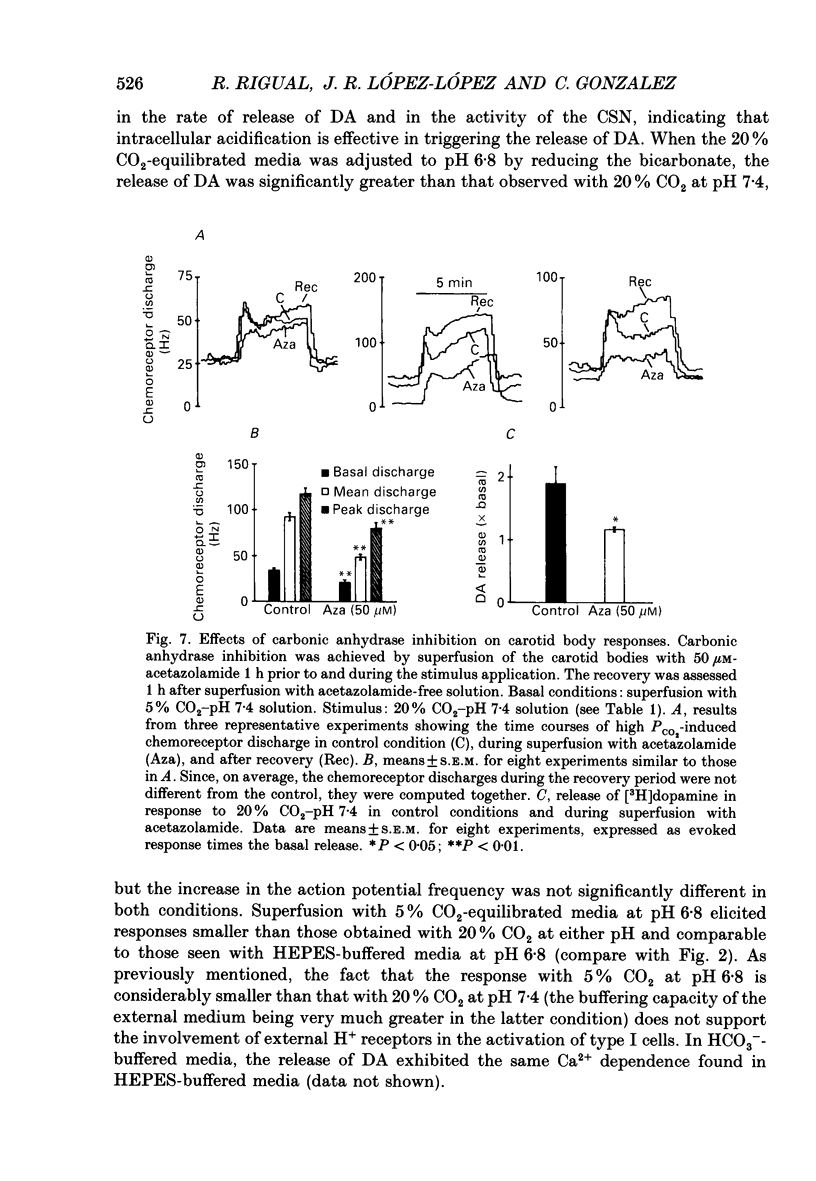
1. Cat carotid bodies were incubated with the precursor [3H]tyrosine to label the catecholamine deposits and then mounted in a superfusion chamber which allowed simultaneous collection of the released [3H]dopamine (DA) and recording of action potentials from the carotid sinus nerve. 2. Low pH (7.2-6.6) superfusion of the carotid bodies for periods of 10 min produced a parallel increase in the release of [3H]DA and chemoreceptor discharge. 3. Carotid sinus nerve denervation of the carotid body 12-15 days prior to the experiments did not modify the release of [3H]DA elicited by low pH. 4. Superfusion of the carotid bodies with Ca(2+)-free, high-Mg2+ (1.6 mM) media reduced basal release of [3H]DA and chemoreceptor discharge by about 30%. Release evoked by low pH was reduced by 82%. Peak and average chemoreceptor discharge recorded in response to low pH were reduced by 28%. 5. Solutions containing weak acids (sodium acetate, 10 mM), adjusted at pH 7.4, elicited release of [3H]DA and increased chemoreceptor discharge. 6. With HCO3-CO2-buffered superfusion media, a reduction of bicarbonate to 5.6 mM (pH 6.8), an increase in CO2 to 20% (pH 6.8), or a simultaneous increase in CO2 to 20% and bicarbonate to 90 mM (pH 7.4), resulted in all cases in a corresponding increase in [3H]DA release and chemoreceptor discharge. The most effective stimulus was 20% CO2-pH 6.8 and the least effective 5% CO2-5.6 mM-HCO3-pH 6.8. 7. Inhibition of carbonic anhydrase with acetazolamide while perfusing the carotid bodies with a 20% CO2-equilibrated (pH 7.4) solution resulted in comparable reductions in the release of [3H]DA and chemoreceptor discharge. 8. It is concluded that the effective acidic stimulus at the carotid body chemoreceptors is an increase in hydrogen ion concentration in type I cells. It is also concluded that DA plays a critical role in the genesis of carotid sinus nerve discharges.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaraz L., Gonzalez C., Obeso A. Effects of high potassium on the release of [3H]dopamine from the cat carotid body in vitro. J Physiol. 1986 Oct;379:293–307. doi: 10.1113/jphysiol.1986.sp016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol. 1989 Jun;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R. S., Garger P., Hauer M. C., Raff H., Fechter L. Effect of hypoxia and hypercapnia on catecholamine content in cat carotid body. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1408–1413. doi: 10.1152/jappl.1983.54.5.1408. [DOI] [PubMed] [Google Scholar]
- Hanbauer I., Hellstrom S. The regulation of dopamine and noradrenaline in the rat carotid body and its modification by denervation and by hypoxia. J Physiol. 1978 Sep;282:21–34. doi: 10.1113/jphysiol.1978.sp012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato E., Narahashi T. Characteristics of the electrical response to dopamine in neuroblastoma cells. J Physiol. 1982 Dec;333:213–226. doi: 10.1113/jphysiol.1982.sp014450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. Receptor for protons in the membrane of sensory neurons. Brain Res. 1981 Jun 9;214(1):150–154. doi: 10.1016/0006-8993(81)90446-7. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Obeso A., Almaraz L., Gonzalez C. Effects of 2-deoxy-D-glucose on in vitro cat carotid body. Brain Res. 1986 Apr 16;371(1):25–36. doi: 10.1016/0006-8993(86)90806-1. [DOI] [PubMed] [Google Scholar]
- Obeso A., Almaraz L., Gonzalez C. Effects of cyanide and uncouplers on chemoreceptor activity and ATP content of the cat carotid body. Brain Res. 1989 Mar 6;481(2):250–257. doi: 10.1016/0006-8993(89)90801-9. [DOI] [PubMed] [Google Scholar]
- Rigual R., Gonzalez E., Fidone S., Gonzalez C. Effects of low pH on synthesis and release of catecholamines in the cat carotid body in vitro. Brain Res. 1984 Aug 20;309(1):178–181. doi: 10.1016/0006-8993(84)91026-6. [DOI] [PubMed] [Google Scholar]
- Rigual R., Gonzalez E., Gonzalez C., Fidone S. Synthesis and release of catecholamines by the cat carotid body in vitro: effects of hypoxic stimulation. Brain Res. 1986 May 21;374(1):101–109. doi: 10.1016/0006-8993(86)90398-7. [DOI] [PubMed] [Google Scholar]
- Rigual R., Iñiguez C., Carreres J., Gonzalez C. Carbonic anhydrase in the carotid body and the carotid sinus nerve. Histochemistry. 1985;82(6):577–580. doi: 10.1007/BF00489979. [DOI] [PubMed] [Google Scholar]
- Rocher A., Obeso A., Gonzalez C., Herreros B. Ionic mechanisms for the transduction of acidic stimuli in rabbit carotid body glomus cells. J Physiol. 1991 Feb;433:533–548. doi: 10.1113/jphysiol.1991.sp018442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Starlinger H., Acker H. The norepinephrine and dopamine content of the cat carotid body in vivo under normoxic and hypoxic conditions. Neurosci Lett. 1986 Feb 14;64(1):65–68. doi: 10.1016/0304-3940(86)90664-6. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance R. W. Convergence of stimuli in arterial chemoreceptors. Adv Exp Med Biol. 1977;78:203–207. doi: 10.1007/978-1-4615-9035-4_16. [DOI] [PubMed] [Google Scholar]
- Van Buskirk R., Dowling J. E. Calcium alters the sensitivity of intact horizontal cells to dopamine antagonists. Proc Natl Acad Sci U S A. 1982 May;79(10):3350–3354. doi: 10.1073/pnas.79.10.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H. The chemical estimation of catecholamines and their metabolites in body fluids and tissue extracts. Methods Biochem Anal. 1971;(Suppl):119–152. doi: 10.1002/9780470110409.ch5. [DOI] [PubMed] [Google Scholar]


