Abstract
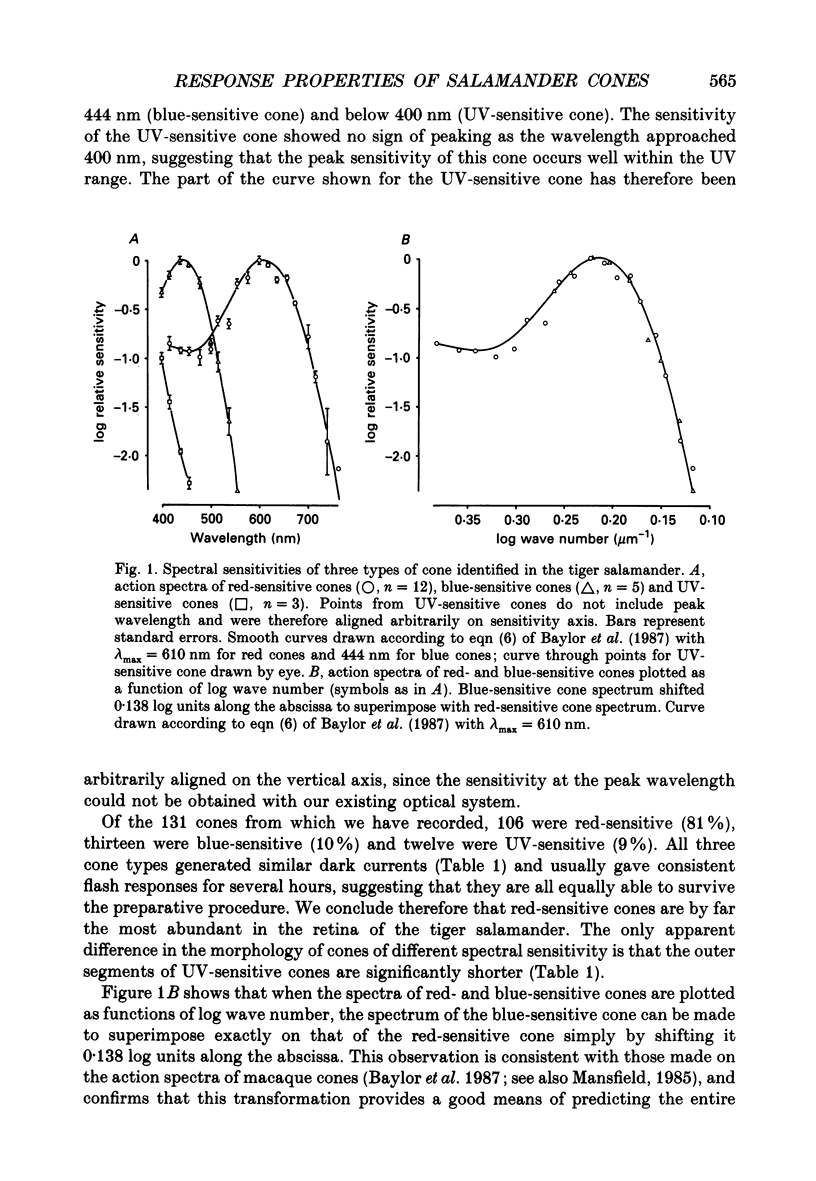
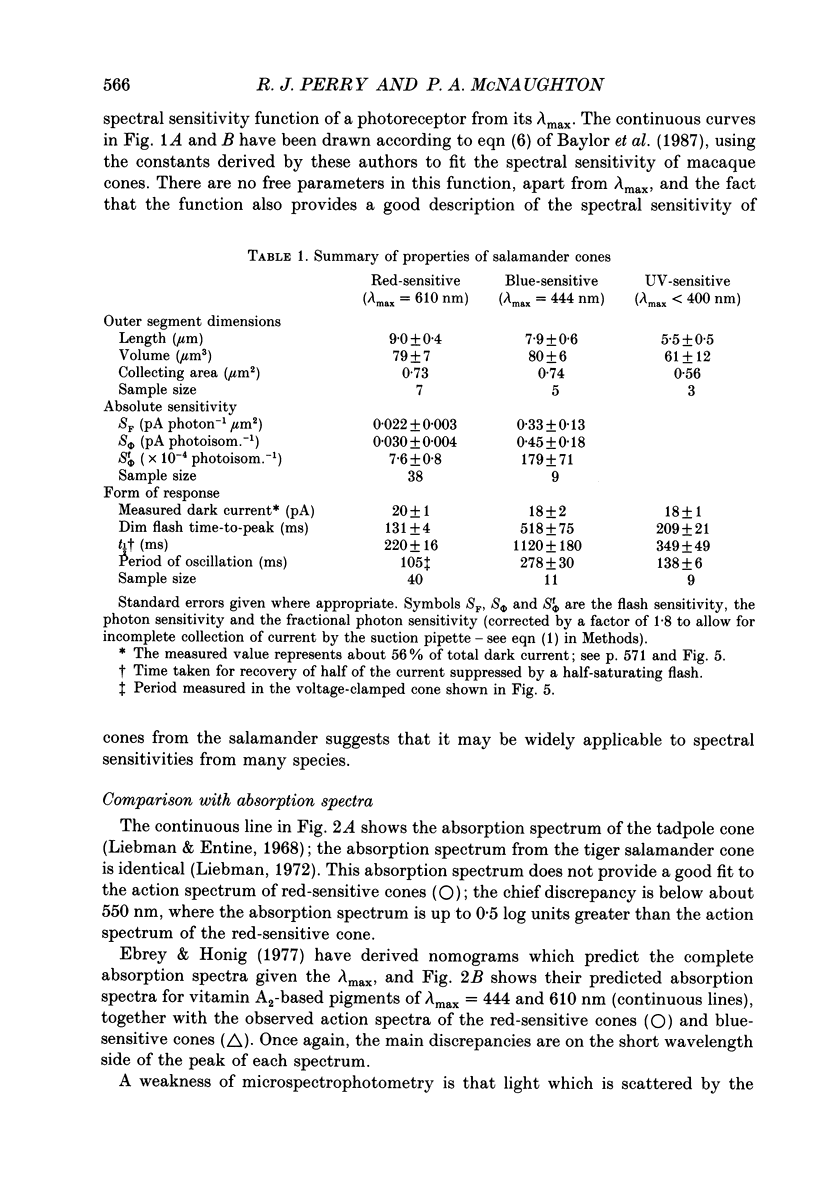
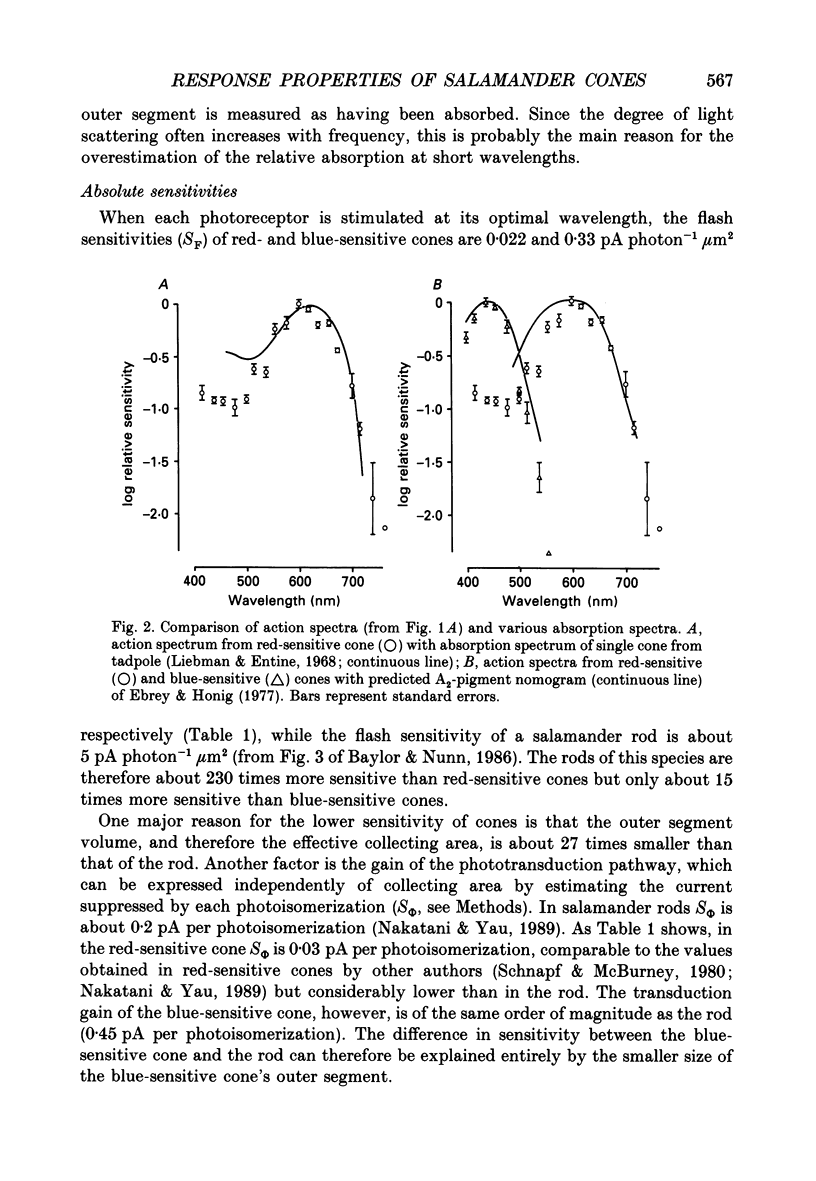
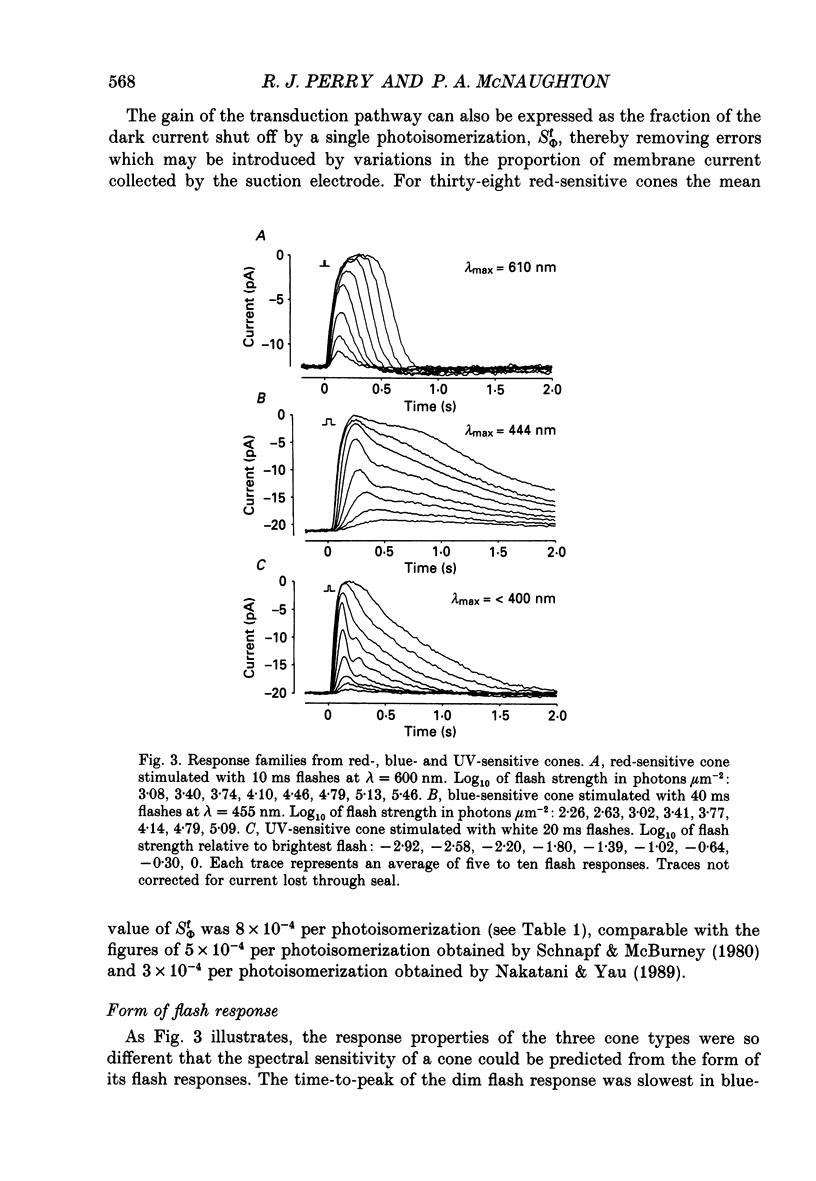
1. Spectral sensitivity measurements using the suction electrode technique reveal three types of cone in the retina of the tiger salamander, showing maximum sensitivity at wavelengths 610 nm (red-sensitive cone), 444 nm (blue-sensitive cone) and below 400 nm (UV-sensitive cone). 2. The absolute sensitivities of red- and blue-sensitive cones to flashes of optimal wavelength are 0.022 and 0.33 pA photon-1 micron 2 respectively. 3. The time-to-peak of the dim flash response and the recovery of membrane current after a flash of any intensity are fastest in red-sensitive and slowest in blue-sensitive cones. 4. In blue- and UV-sensitive cones the flash response peaks progressively earlier as the flash strength is increased, as in rods. In red-sensitive cones, however, bright flash responses take longer to peak than dim flash responses. 5. In all three cone types, voltage clamping at -40 mV reduces the time-to-peak of the response to a bright flash, showing that the rising phase of the bright flash response is normally limited by the time constant of the cell. Under voltage clamp, all cones show a decrease in time-to-peak with increasing flash intensity. 6. Voltage clamping red-sensitive cones reveals two components of the rising phase of the response to a bright flash. Most of the current is rapidly suppressed by a bright flash, and represents the closure of light-sensitive channels. The residual current decays with a mean time constant of 20 ms, and is probably attributable to the decline of electrogenic Na(+)-Ca2+, K+ exchange. The amplitude of this exchange current suggests that the proportion of the dark current carried by calcium ions is greater in red-sensitive cones than in rods of the same species. 7. In UV-sensitive cones, a prominent oscillation of light-sensitive current is observed during the recovery from flashes of intermediate intensity. A similar, but slower and less prominent oscillation is usually seen in blue-sensitive cones. 8. When a red-sensitive cone is voltage clamped an oscillation similar to those in the other two cone types is revealed. An underswing of up to 2 pA is also observed after recovery from intermediate or bright flashes in the majority of red-sensitive cones, and voltage clamping increases the amplitude of this underswing.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







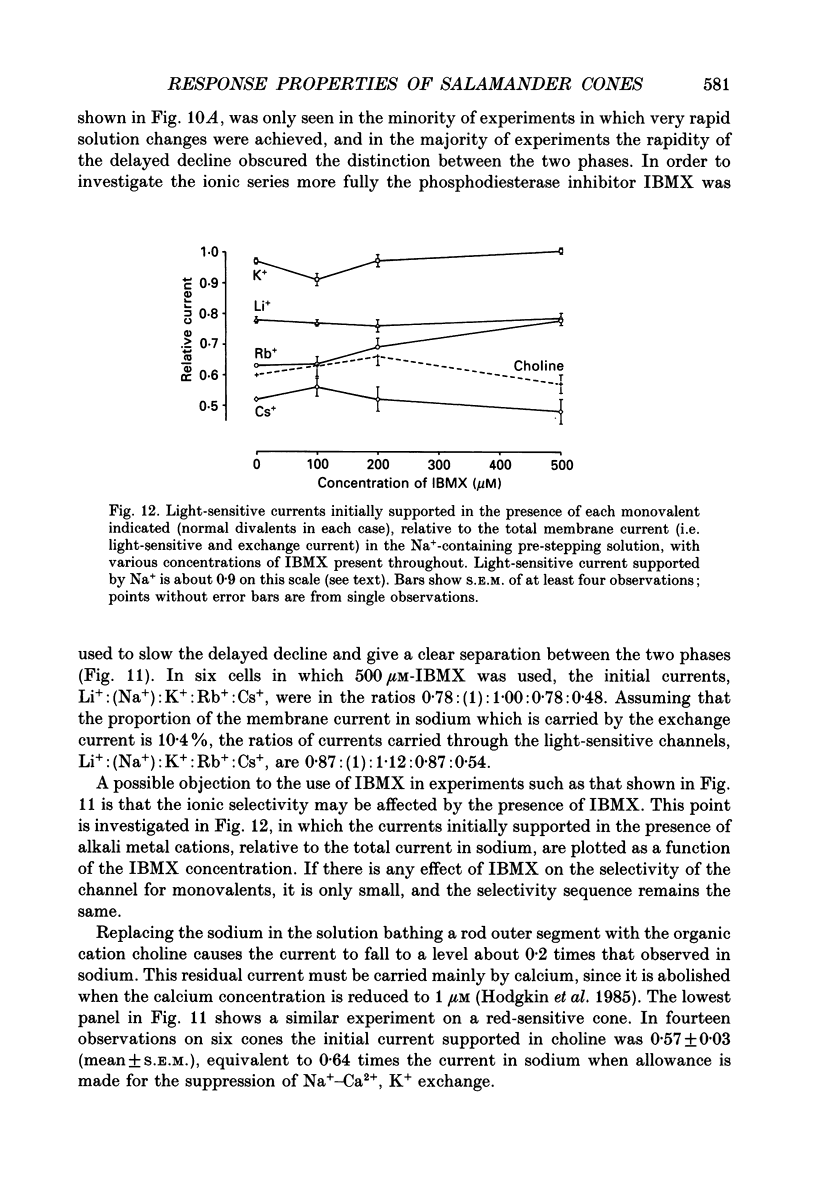
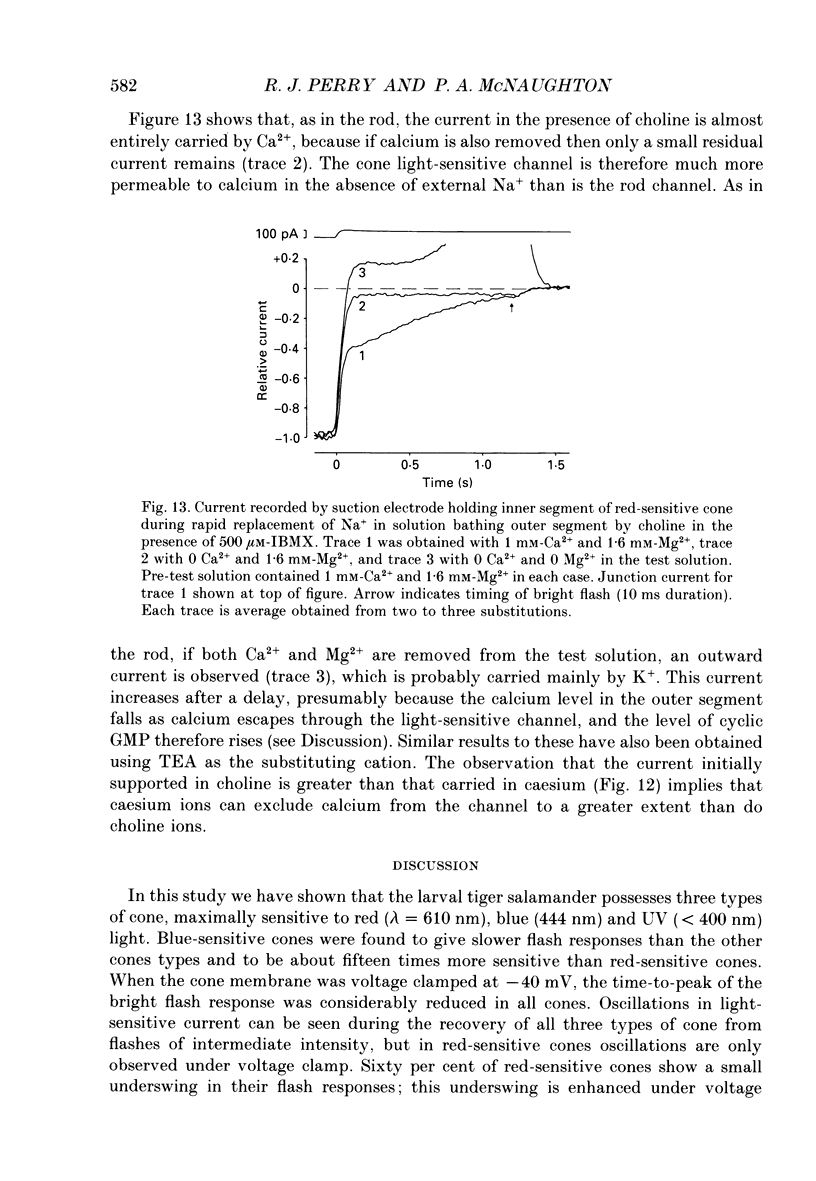





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Werblin F. S., Wilson M. The properties of single cones isolated from the tiger salamander retina. J Physiol. 1982 Jul;328:259–283. doi: 10.1113/jphysiol.1982.sp014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S., Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol. 1989 Oct;94(4):719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D. Local effects of bleaching in retinal rods of the toad. J Physiol. 1982 Jul;328:49–71. doi: 10.1113/jphysiol.1982.sp014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987 Sep;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Kunz Y. W. Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout (Salmo trutta): age-dependent changes. Vision Res. 1987;27(12):2101–2108. doi: 10.1016/0042-6989(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P. A. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986 Jan;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs W. H., Barkdoll A. E., 3rd, Pugh E. N., Jr Cyclic GMP increases photocurrent and light sensitivity of retinal cones. Nature. 1985 Sep 5;317(6032):64–66. doi: 10.1038/317064a0. [DOI] [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. J Physiol. 1987 Dec;394:529–572. doi: 10.1113/jphysiol.1987.sp016884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., MacNichol E. F., Jr, Fein A. Absorptance and spectral sensitivity measurements of rod photoreceptors of the tiger salamander, Ambystoma tigrinum. Vision Res. 1984;24(11):1651–1659. doi: 10.1016/0042-6989(84)90323-7. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. New wavelength dependent visual pigment nomograms. Vision Res. 1977;17(1):147–151. doi: 10.1016/0042-6989(77)90213-9. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Korenbrot J. I. Activation kinetics of retinal cones and rods: response to intense flashes of light. J Neurosci. 1990 Jun;10(6):1967–1973. doi: 10.1523/JNEUROSCI.10-06-01967.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. Control of light-sensitive current in salamander rods. J Physiol. 1988 Sep;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4548–4552. doi: 10.1073/pnas.85.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22(12):1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- McNaughton P. A. Light response of vertebrate photoreceptors. Physiol Rev. 1990 Jul;70(3):847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Sodium-dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J Physiol. 1989 Feb;409:525–548. doi: 10.1113/jphysiol.1989.sp017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf J. L., Kraft T. W., Baylor D. A. Spectral sensitivity of human cone photoreceptors. 1987 Jan 29-Feb 4Nature. 325(6103):439–441. doi: 10.1038/325439a0. [DOI] [PubMed] [Google Scholar]
- Schnapf J. L., McBurney R. N. Light-induced changes in membrane current in cone outer segments of tiger salamander and turtle. Nature. 1980 Sep 18;287(5779):239–241. doi: 10.1038/287239a0. [DOI] [PubMed] [Google Scholar]
- Stark W. S., Tan K. E. Ultraviolet light: photosensitivity and other effects on the visual system. Photochem Photobiol. 1982 Sep;36(3):371–380. doi: 10.1111/j.1751-1097.1982.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]


