Abstract
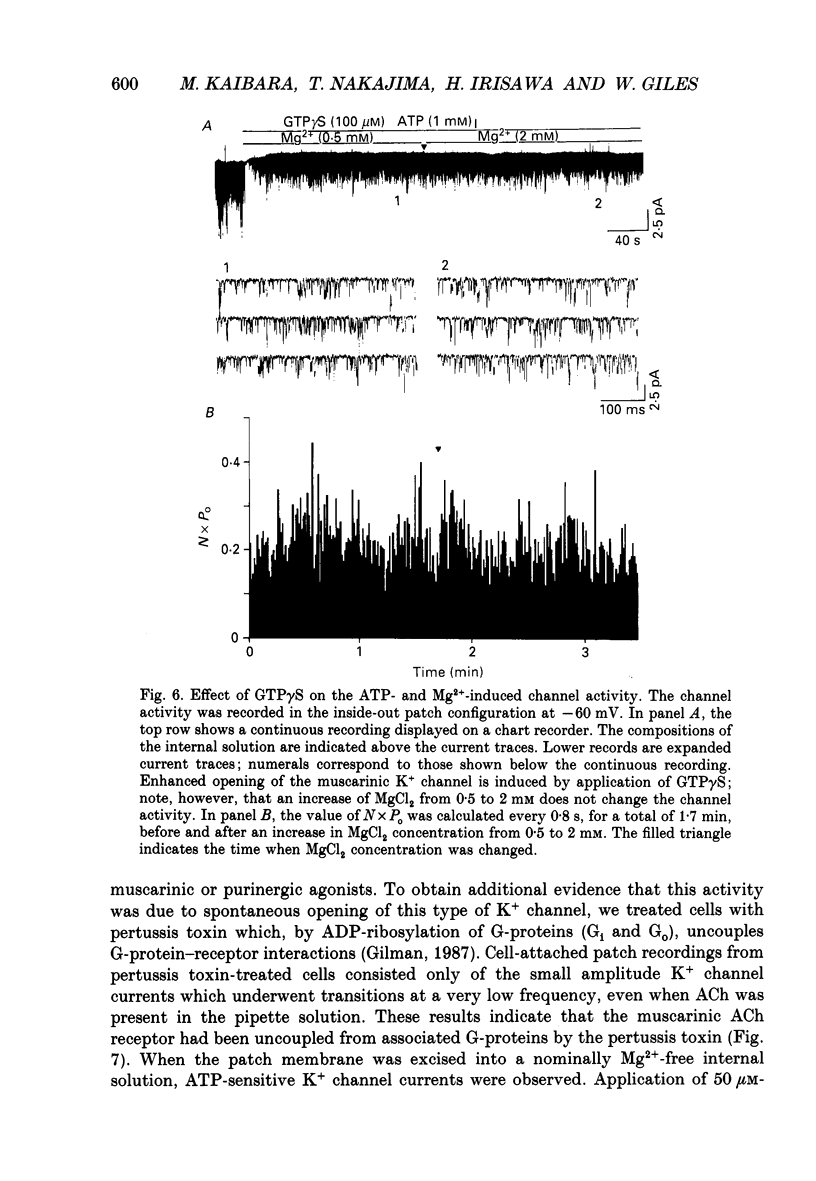
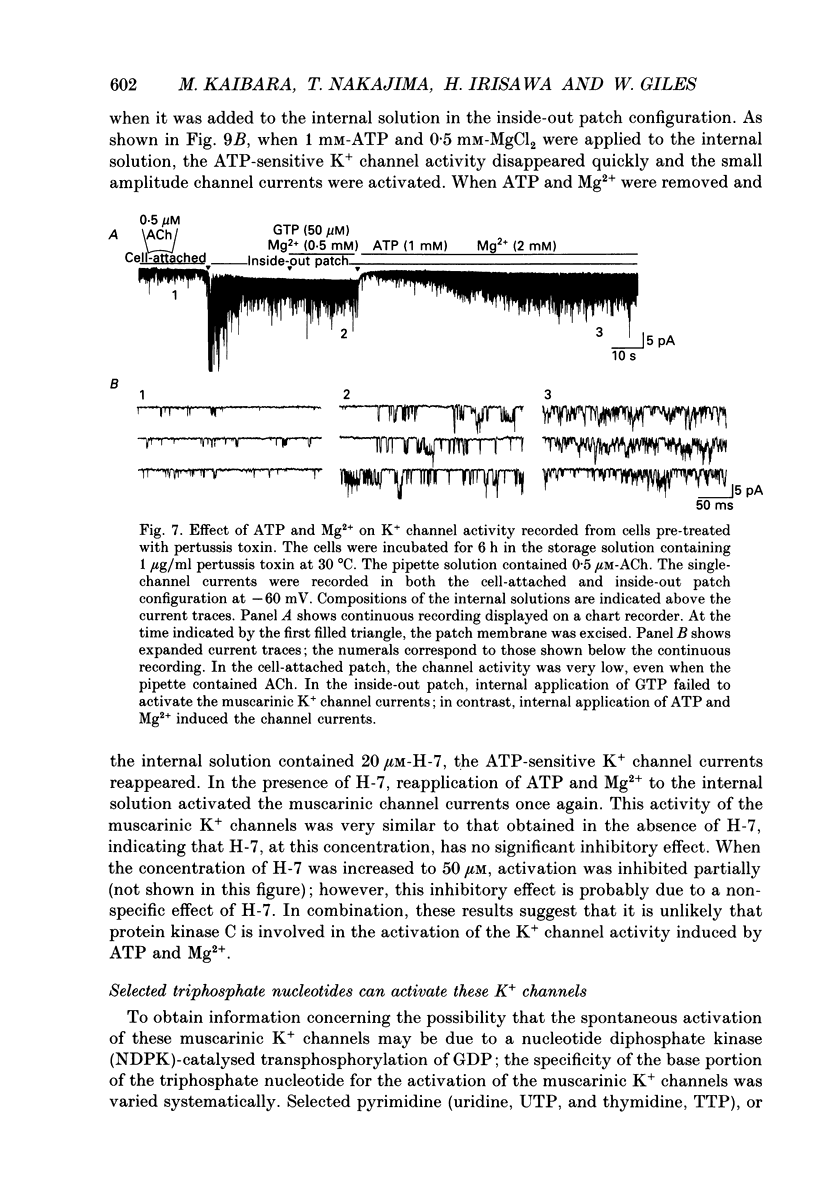
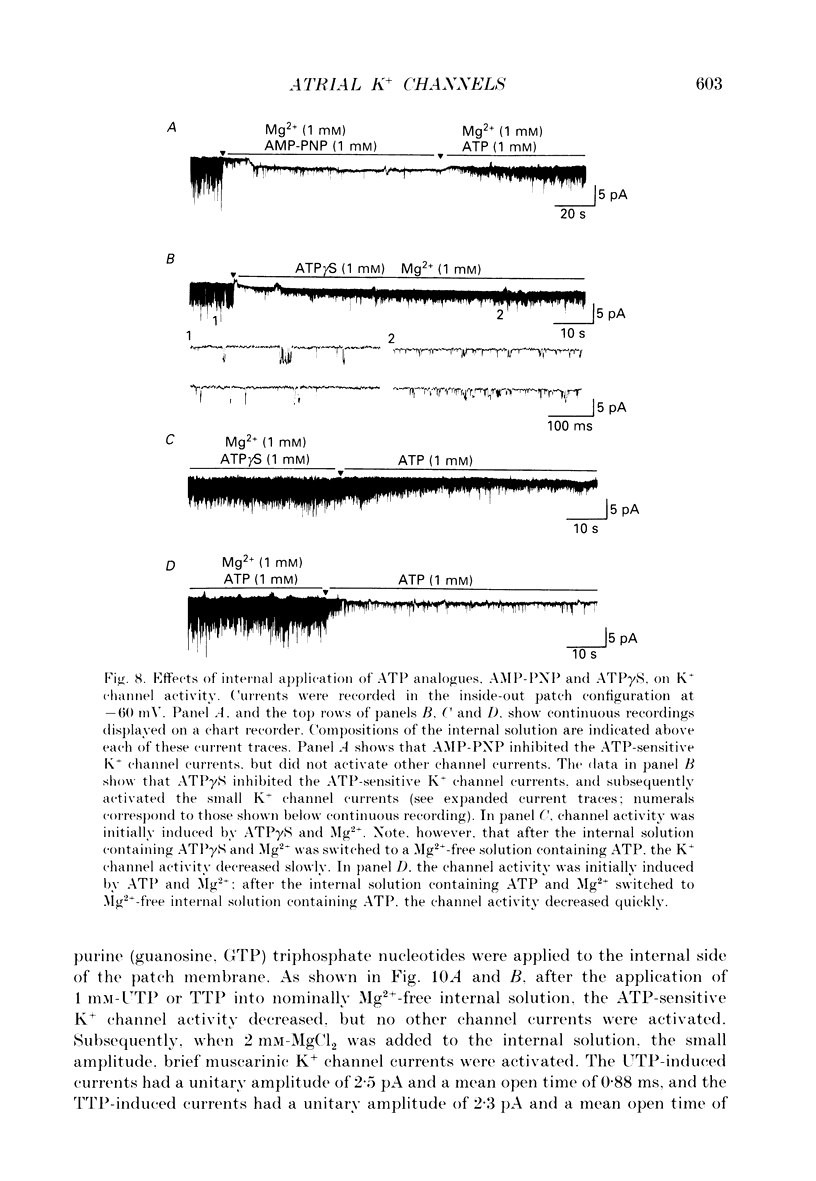
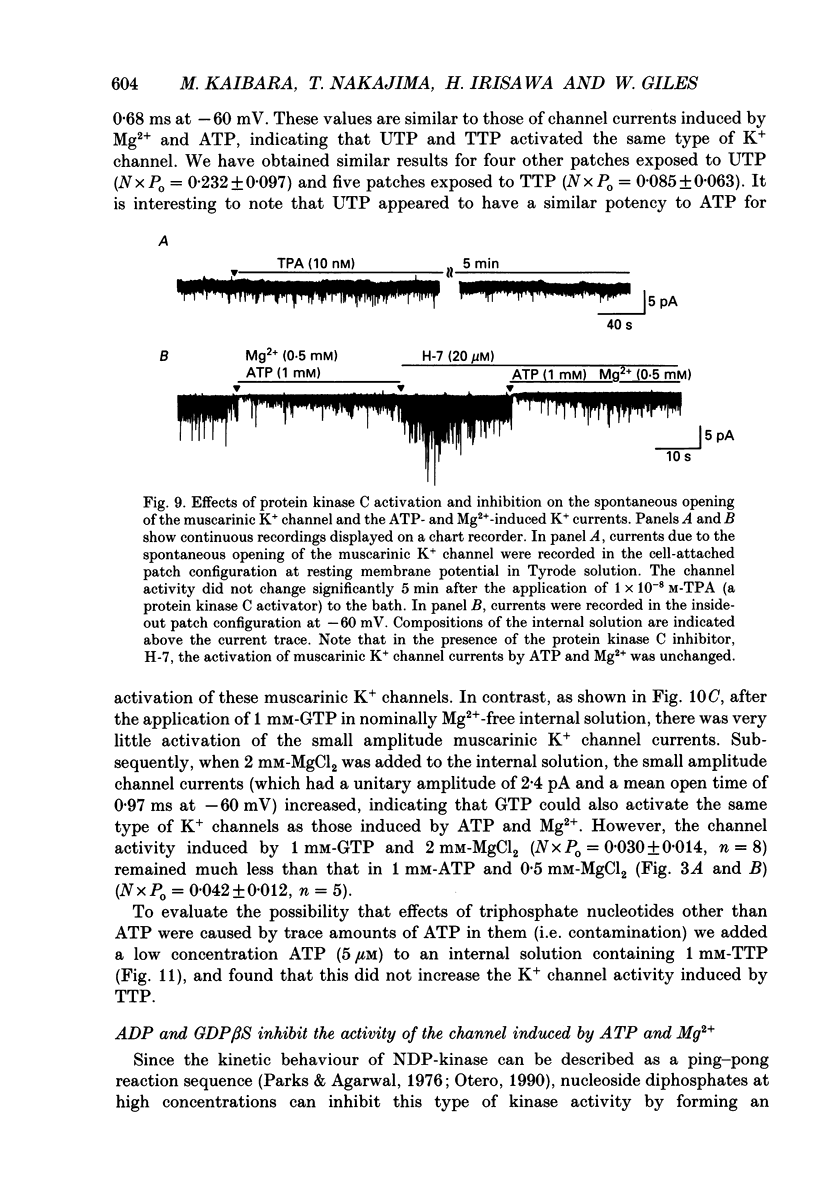
1. Intracellular mechanism(s) for controlling the opening of muscarinic K+ channels in the absence of an applied muscarinic agonist were studied in rabbit atrium by applying the patch clamp technique to isolated single myocytes. 2. In the cell-attached patch configuration, currents due to the activity of both the muscarinic K+ channel and the inward rectifying K+ channel were recorded. However, while the inward rectifying K+ channel currents were observed in only ten patches of 211 examined, spontaneous opening (i.e. in the absence of a muscarinic agonist) of the muscarinic K+ channel currents was observed in all patches examined in these atrial cells. 3. The single-channel currents due to spontaneous opening of muscarinic K+ channels were identified on the basis of their very similar conductance and gating properties to the unitary events which have been recorded when 0.5 microM-acetylcholine is included in the pipette and 10 microM-GTP is present in the internal side of the patch membrane. 4. Although the spontaneous opening of the muscarinic K+ channels disappeared soon after excision of the patch membrane, this type of channel activity reappeared following application of ATP and MgCl2 to the internal side of the torn-off patch, as expected from previous publications. 5. The K+ channel activity induced by the ATP and Mg2+ (measured as the product of the number of channels, N, times the probability of opening, Po) was strongly dependent upon concentration of free Mg2+; it was half-maximal at 2.2 x 10(-4) M [Mg2+]i. However, after the muscarinic K+ channels had been activated by 100 microM-guanosine 5'-O-3-thiotriphosphate (GTP gamma S) together with ATP and Mg2+, an increase in the Mg2+ concentration from 5.5 x 10(-5) to 2 x 10(-3) M failed to enhance this channel activity. 6. Pertussis toxin, which is known to uncouple muscarinic receptors from associated G-proteins (G(i) or G(o)), failed to inhibit the ATP- and Mg(2+)-induced activation of this K+ channel in the absence agonists. 7. In experiments made to test whether the Mg(2+)-ATP requirement results from an obligatory phosphorylation reaction, ATP was replaced with adenylyl-imidodiphosphate (AMP-PNP), an analogue of ATP which is resistant to hydrolysis. This K+ channel activity was not present when ATP was replaced with AMP-PNP.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








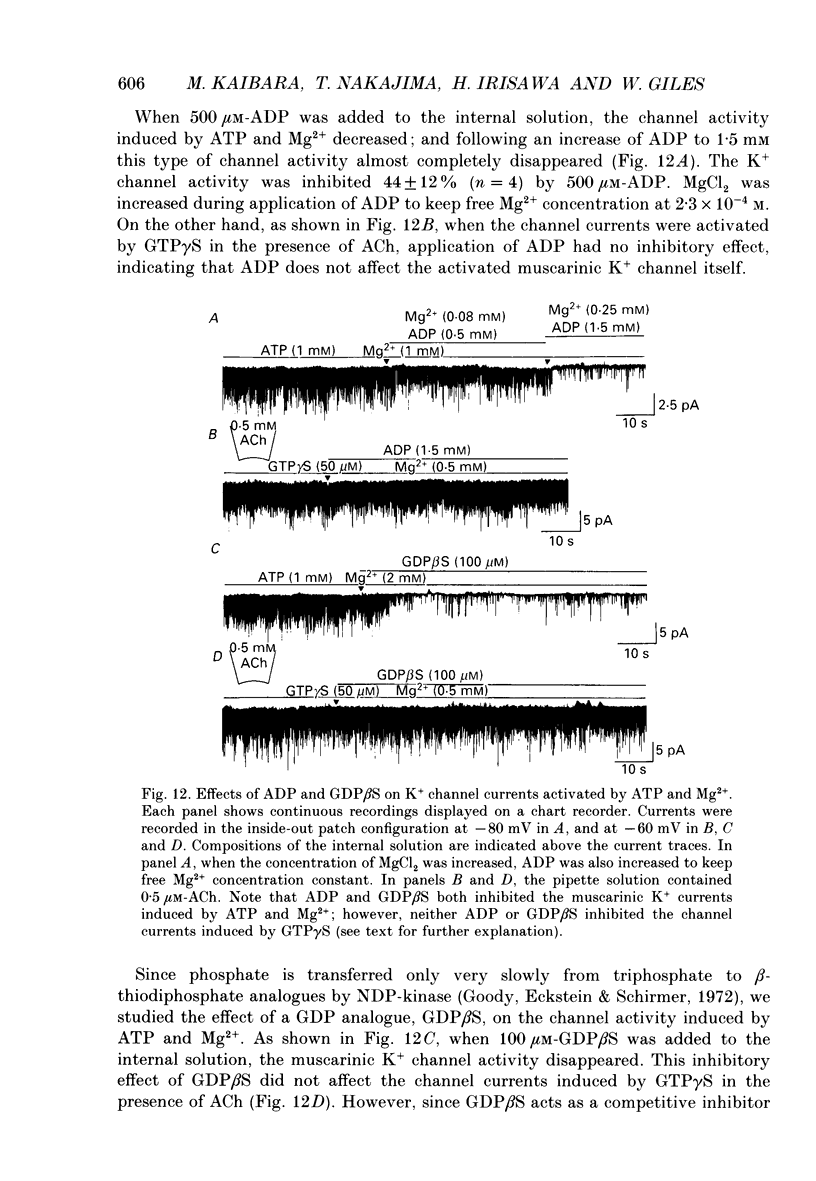
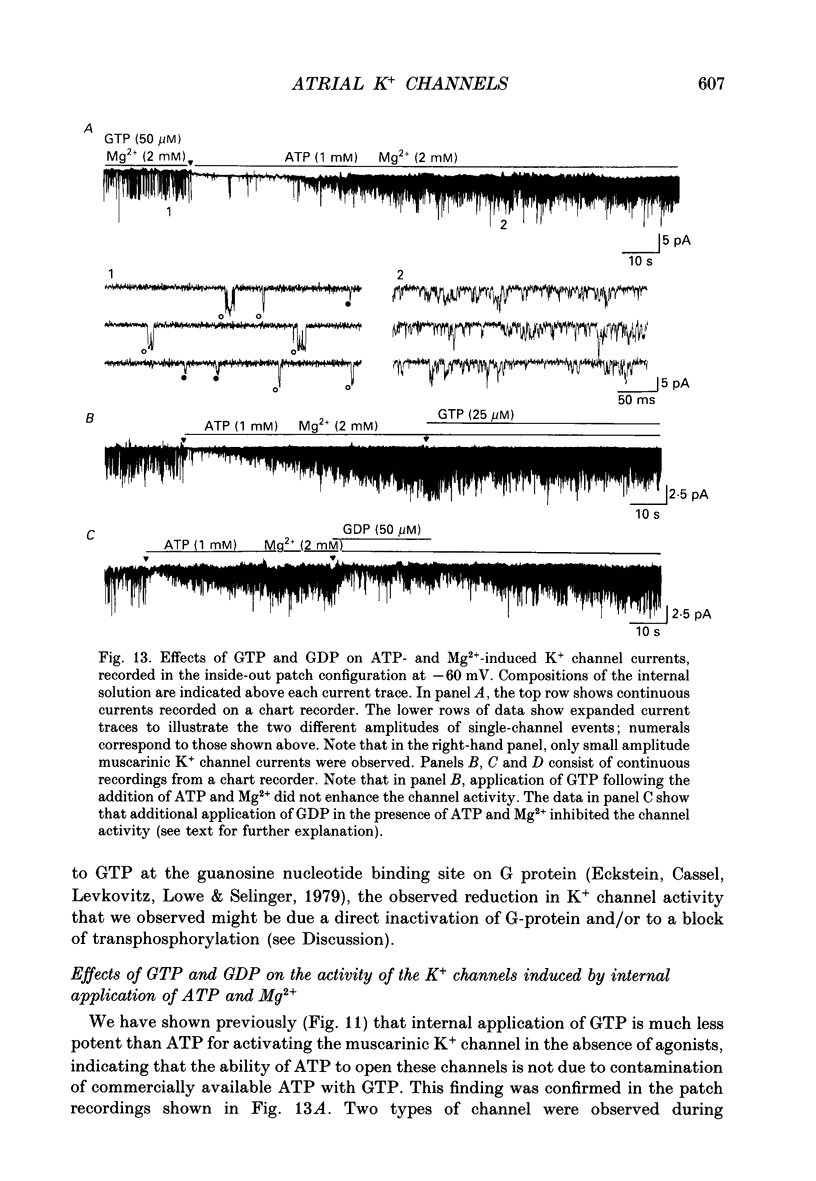






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Giles W. R., West A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol. 1988 Nov;405:615–633. doi: 10.1113/jphysiol.1988.sp017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., McGuigan J. A. Free intracellular magnesium concentration in ferret ventricular muscle measured with ion selective micro-electrodes. Q J Exp Physiol. 1986 Jul;71(3):467–473. doi: 10.1113/expphysiol.1986.sp003005. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Direct G protein gating of ion channels. Am J Physiol. 1988 Mar;254(3 Pt 2):H401–H410. doi: 10.1152/ajpheart.1988.254.3.H401. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Cerbai E., Klöckner U., Isenberg G. The alpha subunit of the GTP binding protein activates muscarinic potassium channels of the atrium. Science. 1988 Jun 24;240(4860):1782–1783. doi: 10.1126/science.2454511. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Giles W. Sodium current in single cells from bullfrog atrium: voltage dependence and ion transfer properties. J Physiol. 1987 Oct;391:235–265. doi: 10.1113/jphysiol.1987.sp016736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Nakajima T., Giles W., Kanai K., Momose Y., Szabo G. Two distinct types of inwardly rectifying K+ channels in bull-frog atrial myocytes. J Physiol. 1990 May;424:229–251. doi: 10.1113/jphysiol.1990.sp018064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Colomb M. G., Chéruy A., Vignais P. V. Nucleoside diphosphokinase from beef heart cytosol. I. Physical and kinetic properties. Biochemistry. 1972 Aug 29;11(18):3370–3378. doi: 10.1021/bi00768a009. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Dual control by ATP and acetylcholine of inwardly rectifying K+ channels in bovine atrial cells. Pflugers Arch. 1990 Mar;415(6):651–657. doi: 10.1007/BF02584001. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Pedersen P. L., Lehninger A. L. The kinetics and inhibition of the adenosine diphosphate-adenosine triphosphate exchange catalyzed by purified mitochondrial nucleoside diphosphokinase. J Biol Chem. 1967 Apr 25;242(8):1845–1853. [PubMed] [Google Scholar]
- Goody R. S., Eckstein F., Schirmer R. H. The enzymatic synthesis of thiophosphate analogs of nucleotides. Biochim Biophys Acta. 1972 Jul 13;276(1):155–161. doi: 10.1016/0005-2744(72)90016-2. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Heidbüchel H., Callewaert G., Vereecke J., Carmeliet E. ATP-dependent activation of atrial muscarinic K+ channels in the absence of agonist and G-nucleotides. Pflugers Arch. 1990 Apr;416(1-2):213–215. doi: 10.1007/BF00370246. [DOI] [PubMed] [Google Scholar]
- Heidbüchel H., Vereecke J., Carmeliet E. Three different potassium channels in human atrium. Contribution to the basal potassium conductance. Circ Res. 1990 May;66(5):1277–1286. doi: 10.1161/01.res.66.5.1277. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Ferguson K. M., Smigel M. D., Gilman A. G. The effect of GTP and Mg2+ on the GTPase activity and the fluorescent properties of Go. J Biol Chem. 1987 Jan 15;262(2):757–761. [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985 Nov;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Kiyosue T., Soejima M. Single channel analysis of the inward rectifier K current in the rabbit ventricular cells. Jpn J Physiol. 1983;33(6):1039–1056. doi: 10.2170/jjphysiol.33.1039. [DOI] [PubMed] [Google Scholar]
- Kimura N., Shimada N. GDP does not mediate but rather inhibits hormonal signal to adenylate cyclase. J Biol Chem. 1983 Feb 25;258(4):2278–2283. [PubMed] [Google Scholar]
- Kimura N., Shimada N. Membrane-associated nucleoside diphosphate kinase from rat liver. Purification, characterization, and comparison with cytosolic enzyme. J Biol Chem. 1988 Apr 5;263(10):4647–4653. [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Acetylcholine activation of K+ channels in cell-free membrane of atrial cells. Am J Physiol. 1986 Sep;251(3 Pt 2):H681–H684. doi: 10.1152/ajpheart.1986.251.3.H681. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Role of intracellular Mg2+ in the activation of muscarinic K+ channel in cardiac atrial cell membrane. Pflugers Arch. 1986 Nov;407(5):572–574. doi: 10.1007/BF00657521. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Murphy E., Freudenrich C. C., Levy L. A., London R. E., Lieberman M. Monitoring cytosolic free magnesium in cultured chicken heart cells by use of the fluorescent indicator Furaptra. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2981–2984. doi: 10.1073/pnas.86.8.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Ohtsuki K., Yokoyama M., Uesaka H. Physiological correlation between nucleoside-diphosphate kinases and the 21-kDa guanine-nucleotide binding proteins copurified with the enzymes from the cell membrane fractions of Ehrlich ascites tumor cells. Biochim Biophys Acta. 1987 Jul 29;929(3):231–238. doi: 10.1016/0167-4889(87)90248-5. [DOI] [PubMed] [Google Scholar]
- Otero A. D. Transphosphorylation and G protein activation. Biochem Pharmacol. 1990 May 1;39(9):1399–1404. doi: 10.1016/0006-2952(90)90420-p. [DOI] [PubMed] [Google Scholar]
- Otero A. S., Breitwieser G. E., Szabo G. Activation of muscarinic potassium currents by ATP gamma S in atrial cells. Science. 1988 Oct 21;242(4877):443–445. doi: 10.1126/science.3051383. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Sekiguchi K., Nishizuka Y. Modulation of ion channel activity: a key function of the protein kinase C enzyme family. Pharmacol Rev. 1989 Jun;41(2):211–237. [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Sorota S., Hoffman B. F. Role of G proteins in the acetylcholine-induced potassium current of canine atrial cells. Am J Physiol. 1989 Nov;257(5 Pt 2):H1516–H1522. doi: 10.1152/ajpheart.1989.257.5.H1516. [DOI] [PubMed] [Google Scholar]
- Szabo G., Otero A. S. G protein mediated regulation of K+ channels in heart. Annu Rev Physiol. 1990;52:293–305. doi: 10.1146/annurev.ph.52.030190.001453. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Uesaka H., Yokoyama M., Ohtsuki K. Physiological correlation between nucleoside-diphosphate kinase and the enzyme-associated guanine nucleotide binding proteins. Biochem Biophys Res Commun. 1987 Mar 13;143(2):552–559. doi: 10.1016/0006-291x(87)91389-1. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Magnesium ions in cardiac function. Regulator of ion channels and second messengers. Biochem Pharmacol. 1989 Mar 15;38(6):859–867. doi: 10.1016/0006-2952(89)90272-4. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]


