Abstract
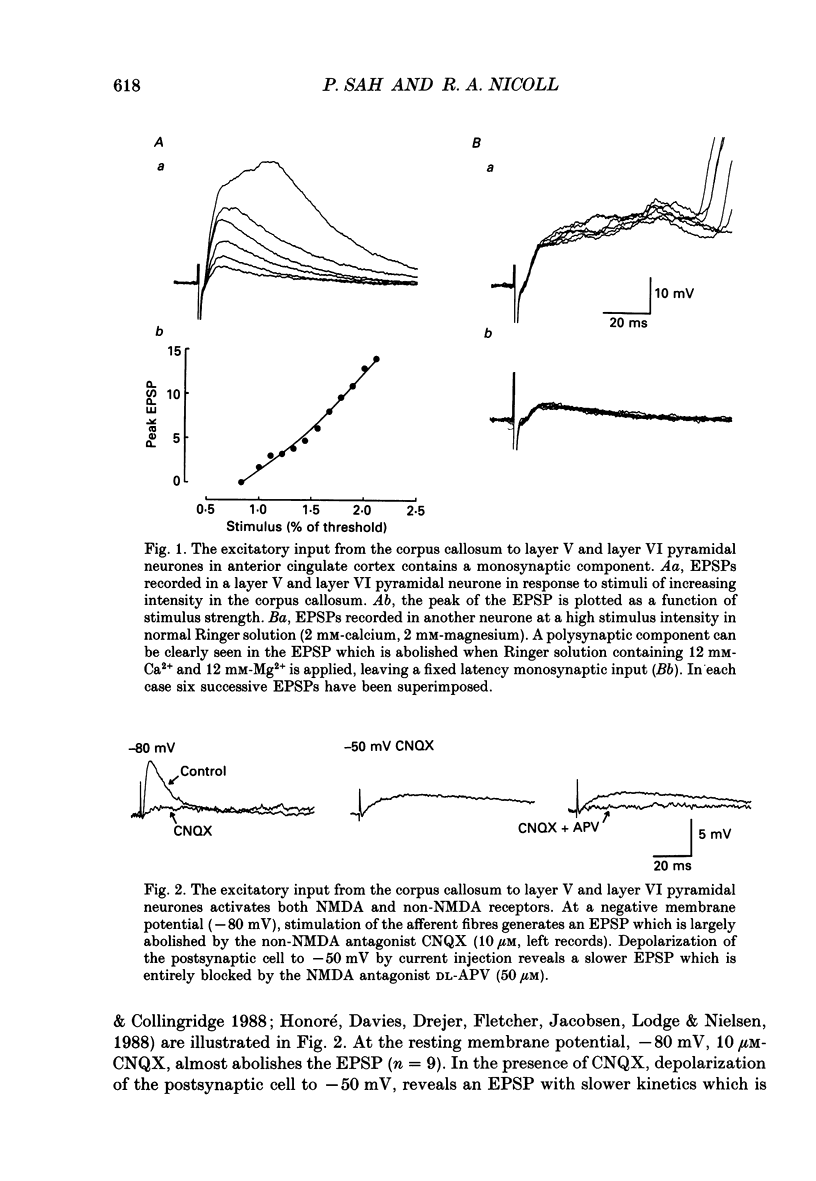
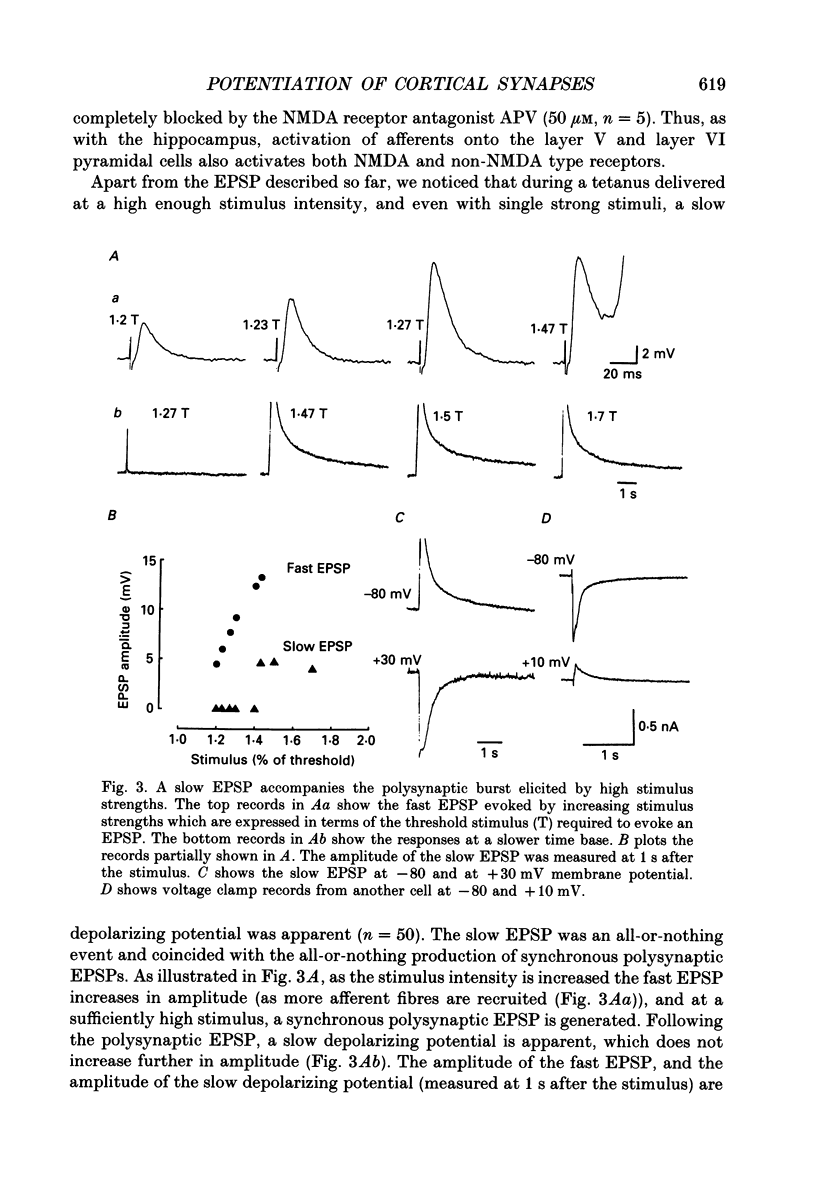
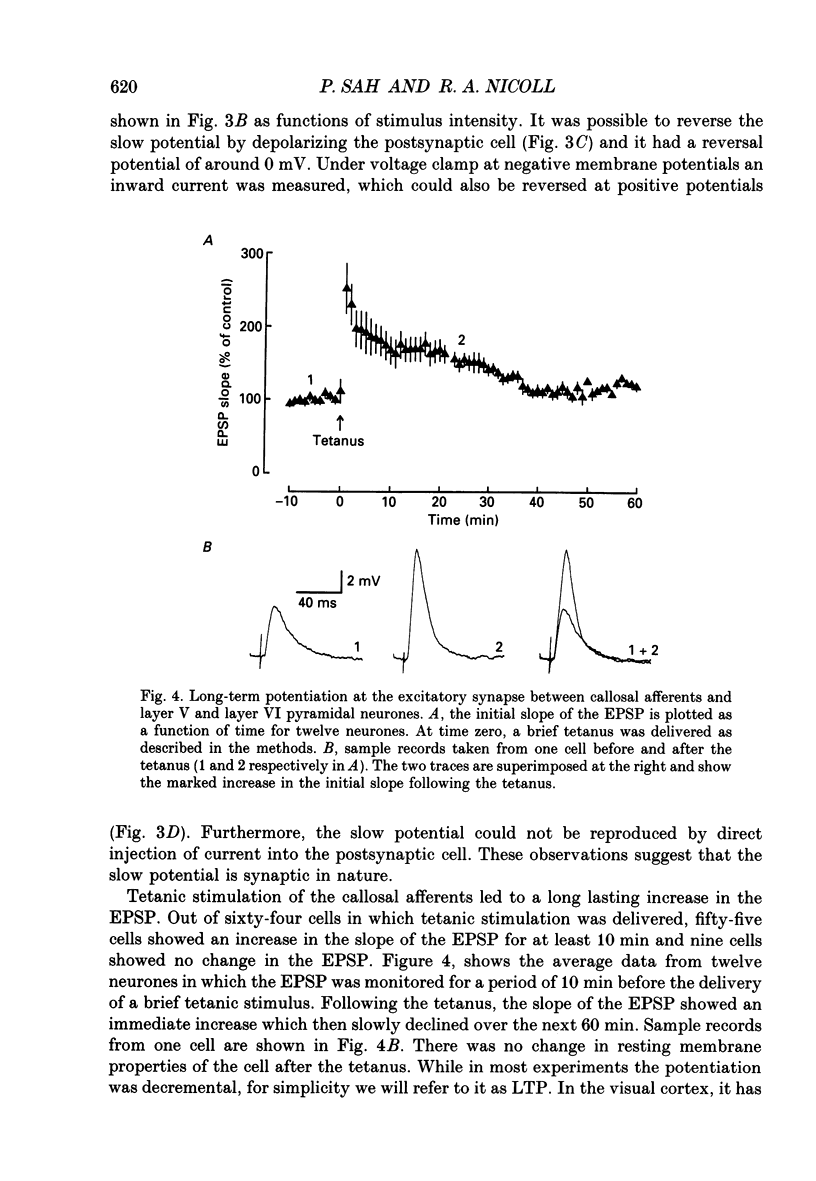
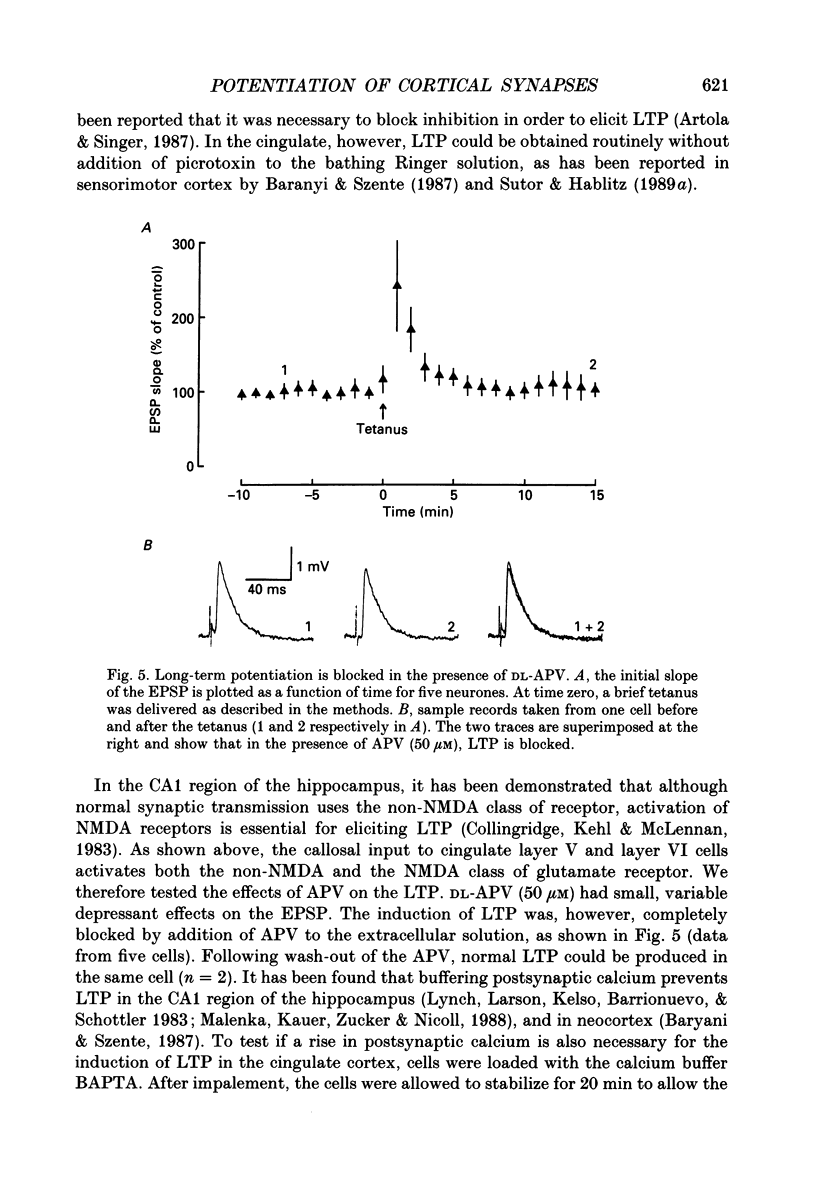
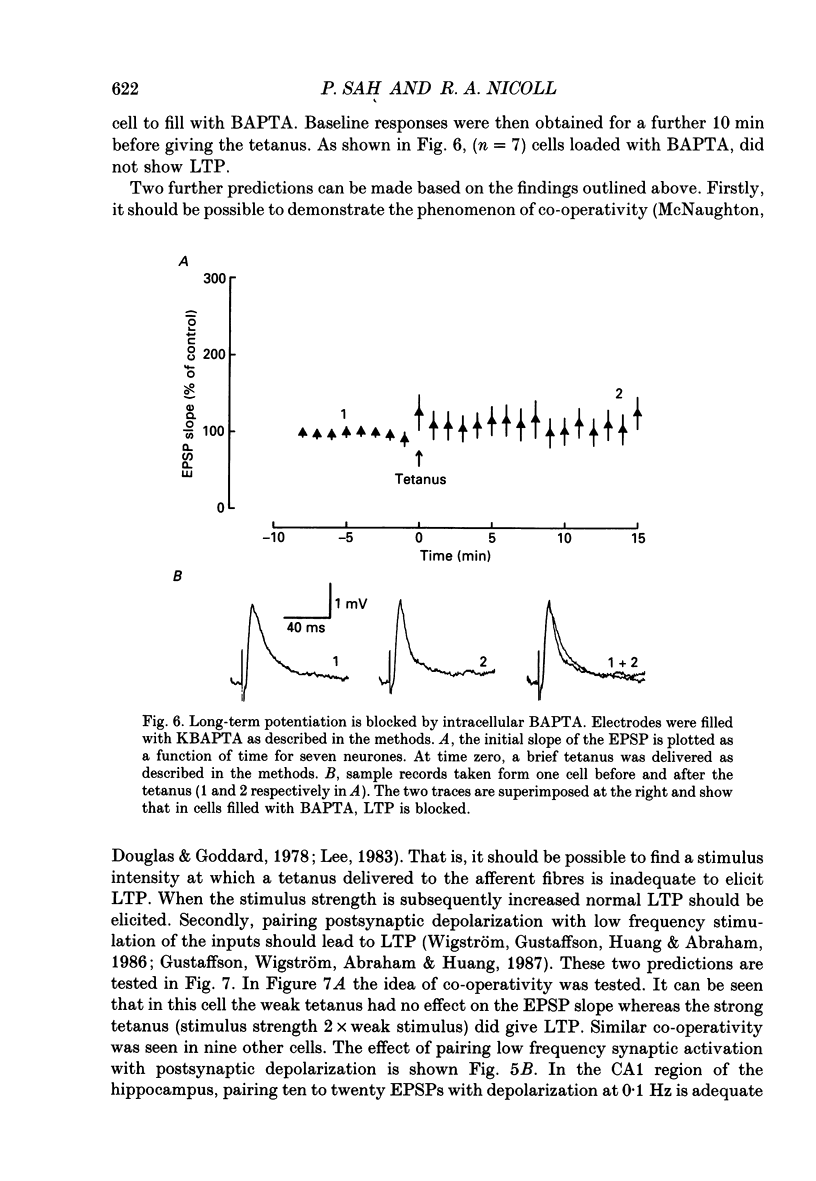
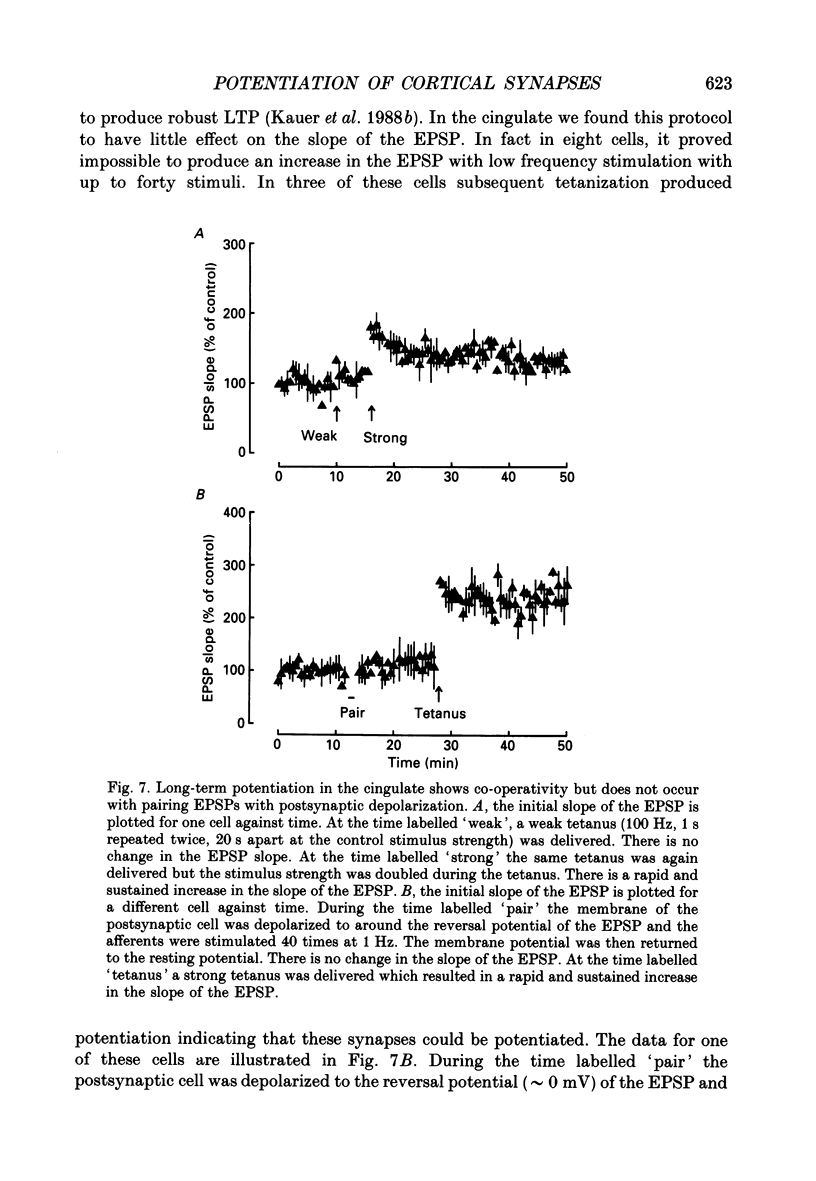
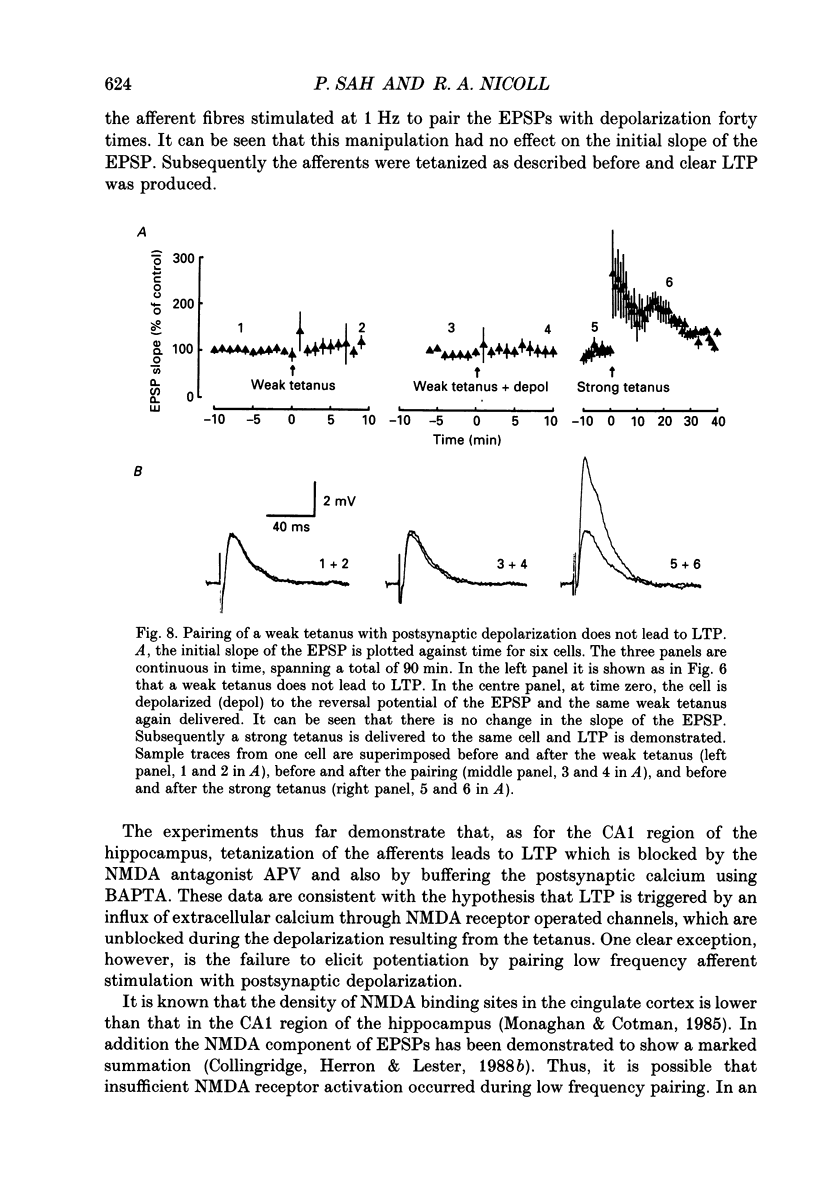
1. The properties of the excitatory synapse made by callosal inputs onto layer V and layer VI cells in the anterior cingulate cortex were studied in an in vitro slice preparation with intracellular recording. 2. In the presence of picrotoxin, the excitatory postsynaptic potential (EPSP) had two components, a fast component blocked by the non-N-methyl-D-aspartate (NMDA) receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and a slow component blocked by the NMDA receptor antagonist DL-2-amino-5-phosphonovalerate (APV). 3. Delivery of a brief tetanus to the afferent fibres led to a long-term potentiation (LTP) of the initial slope of the monosynaptic EPSP. The LTP displayed the property of co-operativity and could be blocked by APV or by buffering intracellular calcium. 4. Pairing of low frequency presynaptic activity or weak tetanic stimulation with postsynaptic depolarization failed to potentiate the EPSP. This suggests that postsynaptic depolarization alone is unable to explain the co-operativity. 5. It is concluded that the transmitter mediating the excitatory input between callosal afferents and layer V and layer VI pyramidal neurones is glutamate. Tetanic stimulation of these afferents leads to LTP which shares many but not all the properties of LTP seen in the CA1 region of the hippocampus.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen M., Lambert J. D., Jensen M. S. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989 Jul;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Baranyi A., Szente M. B. Long-lasting potentiation of synaptic transmission requires postsynaptic modifications in the neocortex. Brain Res. 1987 Oct 13;423(1-2):378–384. doi: 10.1016/0006-8993(87)90867-5. [DOI] [PubMed] [Google Scholar]
- Bindman L. J., Murphy K. P., Pockett S. Postsynaptic control of the induction of long-term changes in efficacy of transmission at neocortical synapses in slices of rat brain. J Neurophysiol. 1988 Sep;60(3):1053–1065. doi: 10.1152/jn.1988.60.3.1053. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Synergism at central synapses due to lateral diffusion of transmitter. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8708–8712. doi: 10.1073/pnas.85.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H., Abraham W. C., Huang Y. Y. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987 Mar;7(3):774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Schultzberg M., Johansson O., Skirboll L., Anggård A., Fredholm B., Hamberger B., Pernow B., Rehfeld J. Cellular localization of peptides in neural structures. Proc R Soc Lond B Biol Sci. 1980 Oct 29;210(1178):63–77. doi: 10.1098/rspb.1980.0119. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. The Ferrier Lecture, 1983. Amino acids and peptides: fast and slow chemical signals in the nervous system? Proc R Soc Lond B Biol Sci. 1984 May 22;221(1224):245–260. doi: 10.1098/rspb.1984.0033. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. Synaptic transmission between dorsal root ganglion and dorsal horn neurons in culture: antagonism of monosynaptic excitatory postsynaptic potentials and glutamate excitation by kynurenate. J Neurosci. 1985 Aug;5(8):2281–2289. doi: 10.1523/JNEUROSCI.05-08-02281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986 Jan;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988 Dec;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988 Jul 21;334(6179):250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Fujii K., Maeda J., Sakaguchi H., Toyama K. Long-term potentiation of synaptic transmission in kitten visual cortex. J Neurophysiol. 1988 Jan;59(1):124–141. doi: 10.1152/jn.1988.59.1.124. [DOI] [PubMed] [Google Scholar]
- Lee K. S. Cooperativity among afferents for the induction of long-term potentiation in the CA1 region of the hippocampus. J Neurosci. 1983 Jul;3(7):1369–1372. doi: 10.1523/JNEUROSCI.03-07-01369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard S., Engberg I., Flatman J. A. The modulation of excitatory amino acid responses by serotonin in the cat neocortex in vitro. Cell Mol Neurobiol. 1987 Dec;7(4):367–379. doi: 10.1007/BF00733789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Kauer J. A., Malenka R. C. The current excitement in long-term potentiation. Neuron. 1988 Apr;1(2):97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perkins A. T., 4th, Teyler T. J. A critical period for long-term potentiation in the developing rat visual cortex. Brain Res. 1988 Jan 26;439(1-2):222–229. doi: 10.1016/0006-8993(88)91478-3. [DOI] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W., Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Rasmusson D. D., Dykes R. W. Long-term enhancement of evoked potentials in cat somatosensory cortex produced by co-activation of the basal forebrain and cutaneous receptors. Exp Brain Res. 1988;70(2):276–286. doi: 10.1007/BF00248353. [DOI] [PubMed] [Google Scholar]
- Reynolds J. N., Baskys A., Carlen P. L. The effects of serotonin on N-methyl-D-aspartate and synaptically evoked depolarizations in rat neocortical neurons. Brain Res. 1988 Jul 26;456(2):286–292. doi: 10.1016/0006-8993(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Porter L. L., Asanuma H. Long-lasting potentiation of synaptic potentials in the motor cortex produced by stimulation of the sensory cortex in the cat: a basis of motor learning. Brain Res. 1987 Jun 16;413(2):360–364. doi: 10.1016/0006-8993(87)91029-8. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S., Nilaver G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide-, and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J Comp Neurol. 1987 Feb 1;256(1):42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., Smith A. D., Nunzi M. G., Gorio A., Wu J. Y. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci. 1984 Oct;4(10):2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling J. S., Patneau D. K., Gramlich C. A. Selective long-term potentiation in the pyriform cortex. Brain Res. 1988 Feb 16;441(1-2):281–291. doi: 10.1016/0006-8993(88)91406-0. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. EPSPs in rat neocortical neurons in vitro. I. Electrophysiological evidence for two distinct EPSPs. J Neurophysiol. 1989 Mar;61(3):607–620. doi: 10.1152/jn.1989.61.3.607. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. EPSPs in rat neocortical neurons in vitro. II. Involvement of N-methyl-D-aspartate receptors in the generation of EPSPs. J Neurophysiol. 1989 Mar;61(3):621–634. doi: 10.1152/jn.1989.61.3.621. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Glycine modulation of the NMDA receptor/channel complex. Trends Neurosci. 1989 Sep;12(9):349–353. doi: 10.1016/0166-2236(89)90042-8. [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Gorman A. L. Responses of cortical neurons to stimulation of corpus callosum in vitro. J Neurophysiol. 1982 Dec;48(6):1257–1273. doi: 10.1152/jn.1982.48.6.1257. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B., Huang Y. Y., Abraham W. C. Hippocampal long-term potentiation is induced by pairing single afferent volleys with intracellularly injected depolarizing current pulses. Acta Physiol Scand. 1986 Feb;126(2):317–319. doi: 10.1111/j.1748-1716.1986.tb07822.x. [DOI] [PubMed] [Google Scholar]


