Abstract
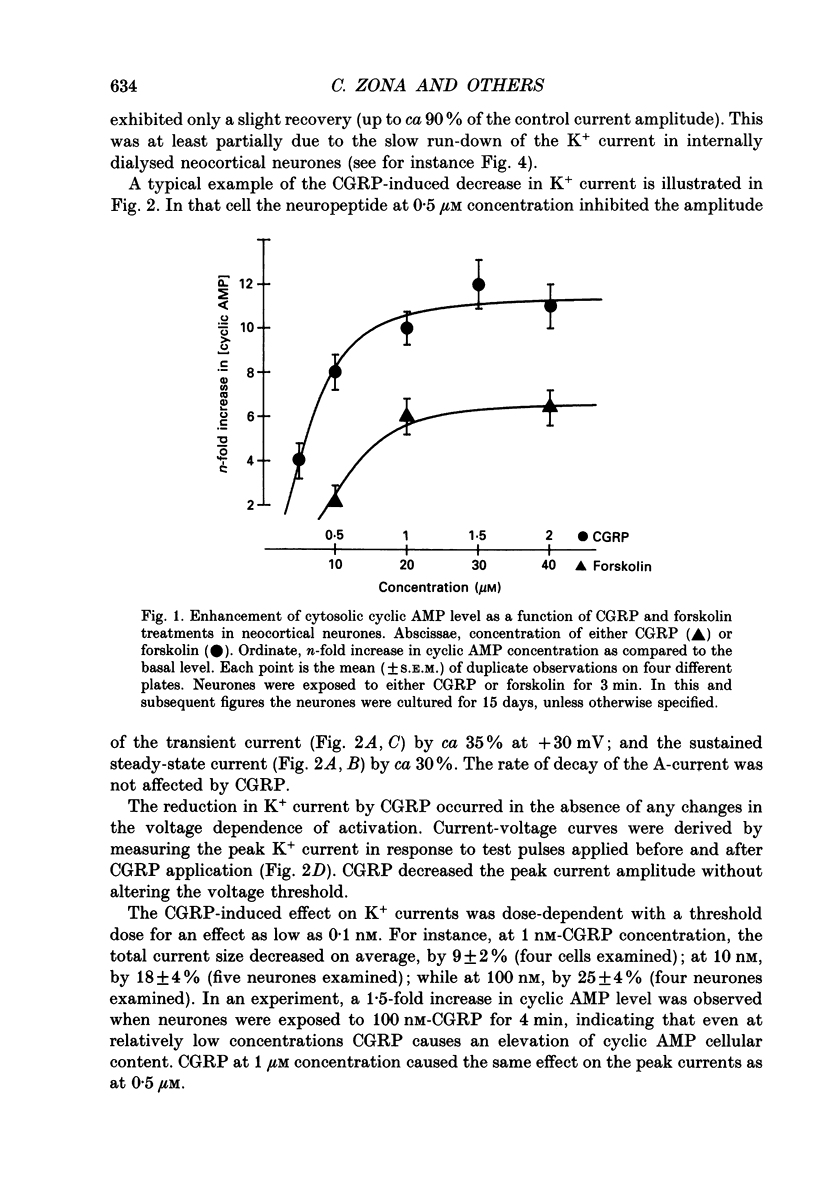
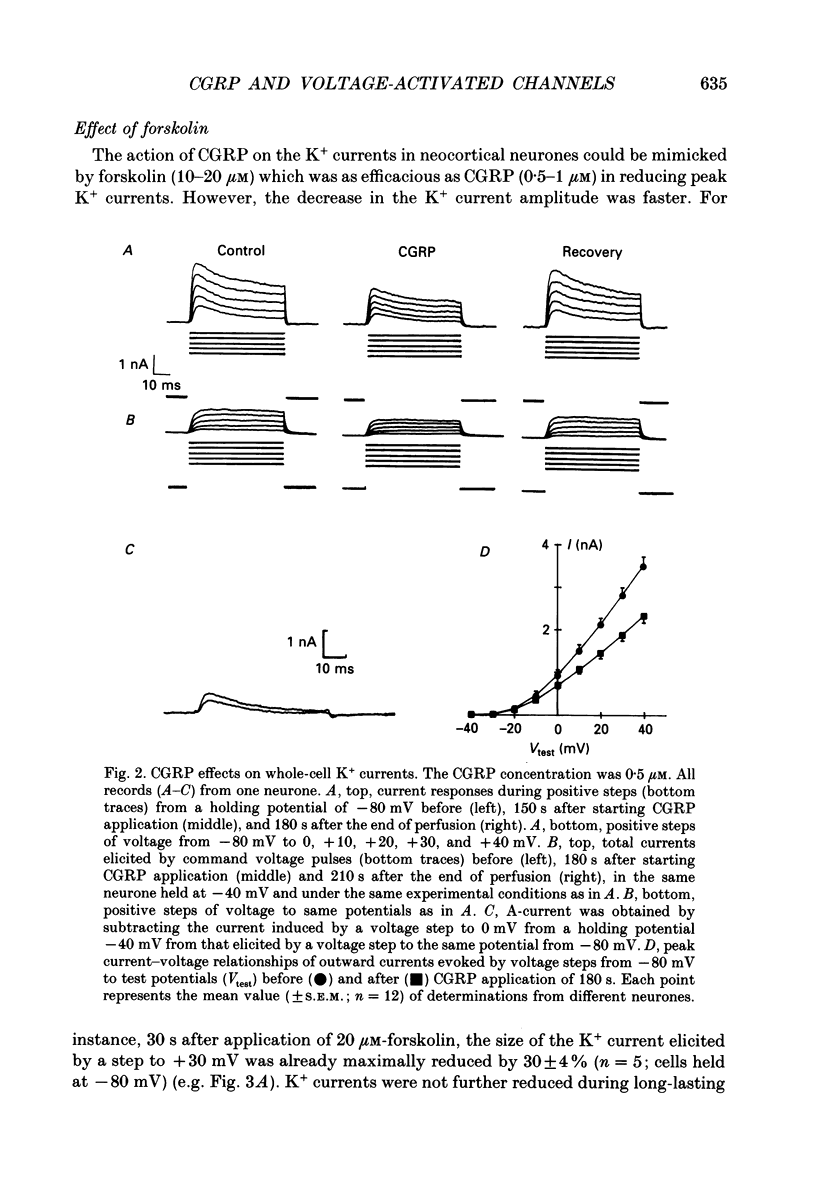
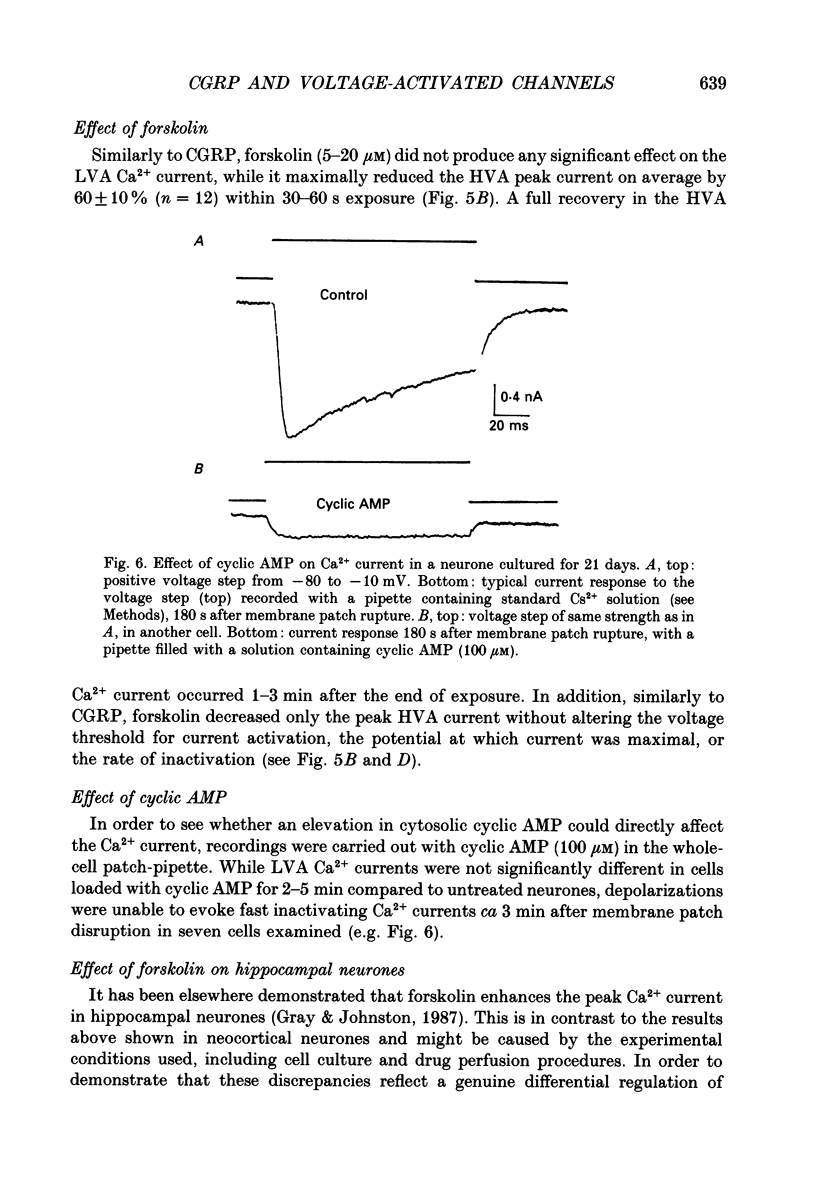
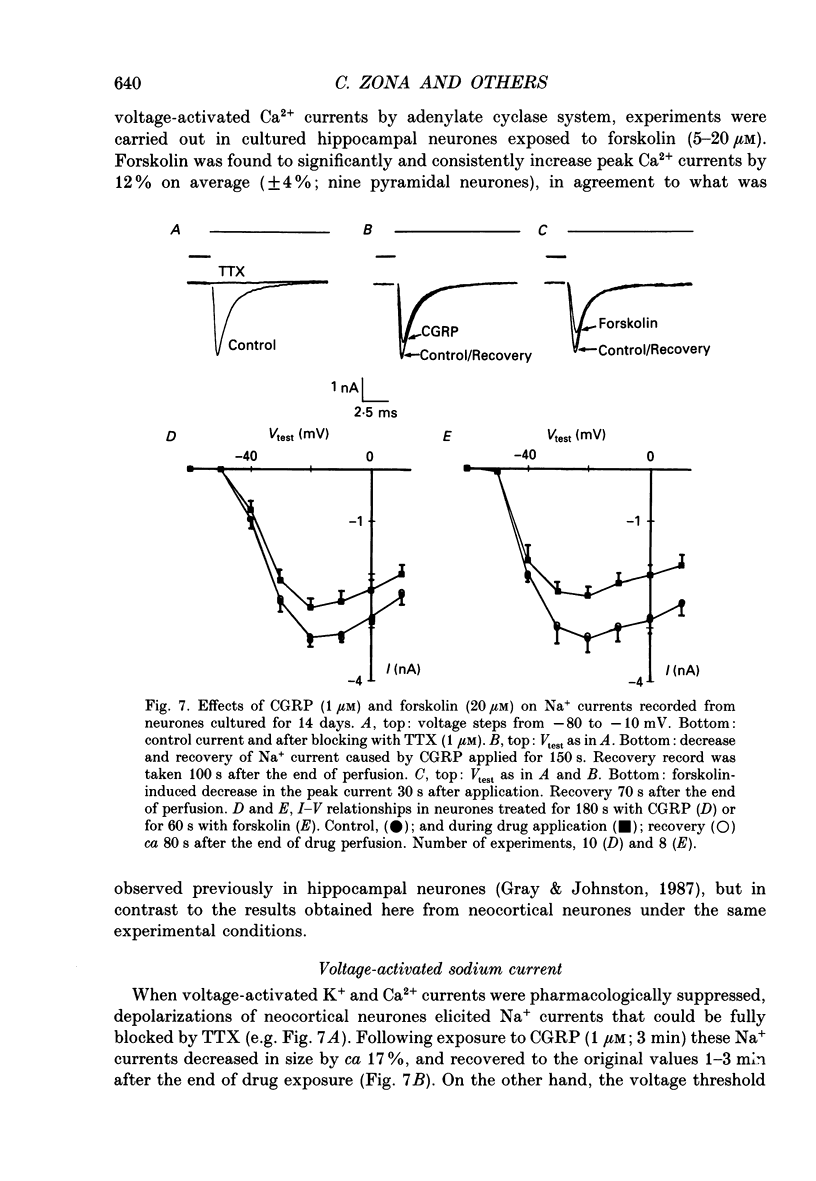
1. Whole-cell currents were recorded from cultures of dissociated neocortical neurones of the rat. Rat alpha-calcitonin gene-related peptide (CGRP; 1 nM-1 microM) caused significant dose-dependent decreases in the voltage-activated transient (A-current) and delayed rectifier K+ currents. Forskolin (10 nM-20 microM) mimicked this effect. Peak K+ currents were gradually decreased after loading neurones with cyclic AMP (100 microM) through patch pipettes. CGRP was ineffective in neurones loaded with cyclic AMP. 2. CGRP (0.5-2 microM) increased cytosolic cyclic AMP concentration and this effect was mimicked by forskolin (5-40 microM). 3. CGRP (0.1-1 microM) reduced high-threshold Ca2+ currents; as did forskolin (5-20 microM) and cyclic AMP loaded into the neurones. In contrast, low-threshold Ca2+ currents were not affected by any of these agents. 4. Voltage-activated Na+ currents were significantly reduced by both CGRP (0.1-1 microM) and forskolin (5-20 microM). A similar effect was observed when cells were loaded with cyclic AMP. 5. We conclude that, in neocortical neurones, CGRP attenuates voltage-activated currents by stimulating the intracellular cyclic AMP signalling system.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browning M. D., Huganir R., Greengard P. Protein phosphorylation and neuronal function. J Neurochem. 1985 Jul;45(1):11–23. doi: 10.1111/j.1471-4159.1985.tb05468.x. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Dichter M. A. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978 Jun 30;149(2):279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Zona C. Calcium currents in cultured rat cortical neurons. Brain Res. 1989 Jul 17;492(1-2):219–229. doi: 10.1016/0006-8993(89)90904-9. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Forskolin prolongs action potential duration and blocks potassium current in embryonic chick sensory neurons. Pflugers Arch. 1985 Feb;403(2):170–174. doi: 10.1007/BF00584096. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Farini D., Grassi F., Monaco L., Ruzzier F. Effects of calcitonin gene-related peptide on synaptic acetylcholine receptor-channels in rat muscle fibres. Proc R Soc Lond B Biol Sci. 1988 Aug 23;234(1276):333–342. doi: 10.1098/rspb.1988.0052. [DOI] [PubMed] [Google Scholar]
- Goltzman D., Mitchell J. Interaction of calcitonin and calcitonin gene-related peptide at receptor sites in target tissues. Science. 1985 Mar 15;227(4692):1343–1345. doi: 10.1126/science.2983422. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida-Yamamoto A., Tohyama M. Calcitonin gene-related peptide in the nervous tissue. Prog Neurobiol. 1989;33(5-6):335–386. doi: 10.1016/0301-0082(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Kriegstein A. R., Dichter M. A. Morphological classification of rat cortical neurons in cell culture. J Neurosci. 1983 Aug;3(8):1634–1647. doi: 10.1523/JNEUROSCI.03-08-01634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K. Toward an understanding of structure and function of ion channels. FASEB J. 1989 Jun;3(8):1906–1914. doi: 10.1096/fasebj.3.8.2470631. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Calcitonin gene-related peptide elevates cyclic AMP levels in chick skeletal muscle: possible neurotrophic role for a coexisting neuronal messenger. EMBO J. 1987 Apr;6(4):901–906. doi: 10.1002/j.1460-2075.1987.tb04836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Mason R. T., Peterfreund R. A., Sawchenko P. E., Corrigan A. Z., Rivier J. E., Vale W. W. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature. 1984 Apr 12;308(5960):653–655. doi: 10.1038/308653a0. [DOI] [PubMed] [Google Scholar]
- Miles K., Greengard P., Huganir R. L. Calcitonin gene-related peptide regulates phosphorylation of the nicotinic acetylcholine receptor in rat myotubes. Neuron. 1989 May;2(5):1517–1524. doi: 10.1016/0896-6273(89)90198-0. [DOI] [PubMed] [Google Scholar]
- Ono K., Delay M., Nakajima T., Irisawa H., Giles W. Calcitonin gene-related peptide regulates calcium current in heart muscle. Nature. 1989 Aug 31;340(6236):721–724. doi: 10.1038/340721a0. [DOI] [PubMed] [Google Scholar]
- Ryu P. D., Gerber G., Murase K., Randic M. Calcitonin gene-related peptide enhances calcium current of rat dorsal root ganglion neurons and spinal excitatory synaptic transmission. Neurosci Lett. 1988 Jul 8;89(3):305–312. doi: 10.1016/0304-3940(88)90544-7. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Change in desensitization of cat muscle acetylcholine receptor caused by coexpression of Torpedo acetylcholine receptor subunits in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):367–371. doi: 10.1073/pnas.86.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toselli M., Lang J., Costa T., Lux H. D. Direct modulation of voltage-dependent calcium channels by muscarinic activation of a pertussis toxin-sensitive G-protein in hippocampal neurons. Pflugers Arch. 1989 Dec;415(3):255–261. doi: 10.1007/BF00370874. [DOI] [PubMed] [Google Scholar]
- Zona C., Pirrone G., Avoli M., Dichter M. Delayed and fast transient potassium currents in rat neocortical neurons in cell culture. Neurosci Lett. 1988 Dec 5;94(3):285–290. doi: 10.1016/0304-3940(88)90032-8. [DOI] [PubMed] [Google Scholar]


